Annual Report 2021
Department of General Internal Medicine
Yoichi Naito, Yasutoshi Kuboki, Tomofumi Miura, Nobuhiko Yamauchi, Kensuke Shinmura, Kazuo Watanabe, Tetsuya Sakai, Saori Mishima
Introduction
We provide general management across all cancer types, support for medical treatment in each department (management of complications, adverse events, etc.), and management aimed at training oncologic specialists. As an educational hospital for the Japanese Society of Internal Medicine, we contribute to training in internal medicine to produce general medical specialists. Based on the experience of immune-related adverse events (irAEs) associated with increasing indications and use of immune checkpoint inhibitors in a wide range of diseases and experience of managing cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) associated with chimeric antigen receptor (CAR) T-cell therapy, we work with all the departments, including the Department of Pharmacy and Department of Nursing, to recognize the occurrence of irAEs, CRS, and ICANS and take countermeasures. We also assist research related to irAEs.
The Team and What We Do
- Increased number of reported irAE, CRS, and ICANS cases (Figure 1)
- Conducting an irAE cooperation seminar
- Revising a manual for immune checkpoint inhibitors
- Developing a manual for drug-induced liver injury and plasma exchange
Figure1. Number of Reported irAE/CRS/ICANS Cases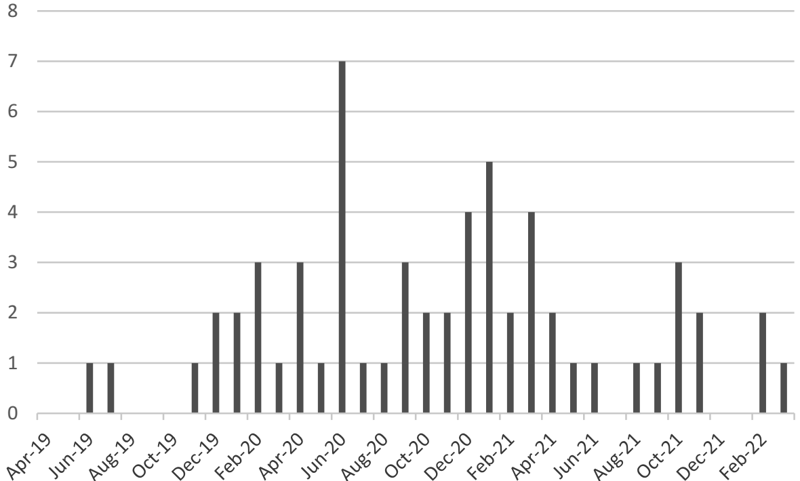

Research activities
- Contributing to training for internal medicine trainees
- Support for trainees presenting cases at the congress of the Japanese Society of Internal Medicine and related congresses
- Support with writing papers
- Intensive lectures on medical oncology education for new residents (updated)
- Support/guidance for new research (Example: Observational study to confirm pharmacokinetics of mycophenolate mofetil for liver damage caused by immune checkpoint inhibitors)
- irAE cooperation seminar with regional institutions
Clinical trials
ical trials One observational study, which we support fully, is currently being planned by the Department of Pharmacy.
Education
We will further collaborate with regional institutions to assemble outstanding trainees, and develop tight connections with several academic institutions. We will revise a manual for immune checkpoint inhibitors, and develop safe and cost-effective practice. We plan to have approximately 100 case consultations on immune-related AEs.
Future Prospects
We will continue high-quality training. The medical treatment for cancer at our hospital is at the top level in Japan, and we will continue to establish a consulting system and improve the medical treatment system so that the valuable experience gained at this hospital can be fed back into general medical treatment. We will continue the irAE cooperation program and promote research planning to identify and improve issues. We will deepen cooperation with the Department of Pharmacy and Department of Nursing, and continue efforts to share and improve issues within the hospital.
We will further focus on management of adverse events caused by various drugs, and on in-hospital and external collaboration. We will prepare a manual on renal failure and hemodialysis, train personnel to become consultants for renal failure, and establish a cooperative system with collaborating facilities.
Regarding education, we will also cooperate with the Human Resource Development Center to enhance internal education and will continuously cooperate with external parties. We will continuously confirm that medical issues can be shared with the risk management department.
List of papers published in 2021
Journal
1. Kumagai S, Koyama S, Itahashi K, Tanegashima T, Lin YT, Togashi Y, Kamada T, Irie T, Okumura G, Kono H, Ito D, Fujii R, Watanabe S, Sai A, Fukuoka S, Sugiyama E, Watanabe G, Owari T, Nishinakamura H, Sugiyama D, Maeda Y, Kawazoe A, Yukami H, Chida K, Ohara Y, Yoshida T, Shinno Y, Takeyasu Y, Shirasawa M, Nakama K, Aokage K, Suzuki J, Ishii G, Kuwata T, Sakamoto N, Kawazu M, Ueno T, Mori T, Yamazaki N, Tsuboi M, Yatabe Y, Kinoshita T, Doi T, Shitara K, Mano H, Nishikawa H. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer cell, 40:201-218.e9, 2022
2. Muro K, Kojima T, Moriwaki T, Kato K, Nagashima F, Kawakami H, Ishihara R, Ogata T, Satoh T, Iwakami K, Han S, Yatsuzuka N, Takami T, Bhagia P, Doi T. Second-line pembrolizumab versus chemotherapy in Japanese patients with advanced esophageal cancer: subgroup analysis from KEYNOTE-181. Esophagus: official journal of the Japan Esophageal Society, 19:137-145, 2022
3. Usui Y, Miura T, Kawaguchi T, Kosugi K, Uehara Y, Kato M, Kosugi T, Sone M, Nakamura N, Mizushima A, Miyashita M, Morita T, Yamaguchi T, Matsumoto Y, Satomi E. Palliative care physicians' recognition of patients after immune checkpoint inhibitors and immune-related adverse events. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer, 30:775-784, 2022
4. Doi T, Kuboki Y, Naito Y, Ishida M, Tanaka T, Takeuchi Y. A phase 1 trial of xentuzumab, an IGF-neutralizing antibody, in Japanese patients with advanced solid tumors. Cancer science, 113:1010-1017, 2022
5. Nakajima H, Harano K, Nakai T, Kusuhara S, Nakao T, Funasaka C, Kondoh C, Matsubara N, Naito Y, Hosono A, Mitsunaga S, Ishii G, Mukohara T. Impacts of clinicopathological factors on efficacy of trastuzumab deruxtecan in patients with HER2-positive metastatic breast cancer. Breast (Edinburgh, Scotland), 61:136-144, 2022
6. Taira N, Kashiwabara K, Tsurutani J, Kitada M, Takahashi M, Kato H, Kikawa Y, Sakata E, Naito Y, Hasegawa Y, Saito T, Iwasa T, Takashima T, Aihara T, Mukai H, Hara F. Quality of life in a randomized phase II study to determine the optimal dose of 3-week cycle nab-paclitaxel in patients with metastatic breast cancer. Breast cancer (Tokyo, Japan), 29:131-143, 2022
7. Taira N, Kashiwabara K, Tsurutani J, Kitada M, Takahashi M, Kato H, Kikawa Y, Sakata E, Naito Y, Hasegawa Y, Saito T, Iwasa T, Takashima T, Aihara T, Mukai H, Hara F. Correction to: Quality of life in a randomized phase II study to determine the optimal dose of 3-week cycle nab-paclitaxel in patients with metastatic breast cancer. Breast cancer (Tokyo, Japan), 29:186-188, 2022
8. Tan RSYC, Ong WS, Lee KH, Lim AH, Park S, Park YH, Lin CH, Lu YS, Ono M, Ueno T, Naito Y, Onishi T, Lim GH, Tan SM, Lee HB, Ryu HS, Han W, Tan VKM, Wong FY, Im SA, Tan PH, Chan JY, Yap YS. HER2 expression, copy number variation and survival outcomes in HER2-low non-metastatic breast cancer: an international multicentre cohort study and TCGA-METABRIC analysis. BMC medicine, 20:105, 2022
9. Diab A, Hamid O, Thompson JA, Ros W, Eskens FALM, Doi T, Hu-Lieskovan S, Klempner SJ, Ganguly B, Fleener C, Wang X, Joh T, Liao K, Salek-Ardakani S, Taylor CT, Chou J, El-Khoueiry AB. A Phase I, Open-Label, Dose-Escalation Study of the OX40 Agonist Ivuxolimab in Patients with Locally Advanced or Metastatic Cancers. Clinical cancer research: an official journal of the American Association for Cancer Research, 28:71-83, 2022
10. Chida K, Kawazoe A, Suzuki T, Kawazu M, Ueno T, Takenouchi K, Nakamura Y, Kuboki Y, Kotani D, Kojima T, Bando H, Mishima S, Kuwata T, Sakamoto N, Watanabe J, Mano H, Ikeda M, Shitara K, Endo I, Nakatsura T, Yoshino T. Transcriptomic Profiling of MSI-H/dMMR Gastrointestinal Tumors to Identify Determinants of Responsiveness to Anti-PD-1 Therapy. Clinical cancer research: an official journal of the American Association for Cancer Research, 28:2110-2117, 2022
11. Shitara K, Doi T, Hosaka H, Thuss-Patience P, Santoro A, Longo F, Ozyilkan O, Cicin I, Park D, Zaanan A, Pericay C, Özgüroğlu M, Alsina M, Makris L, Benhadji KA, Ilson DH . Efficacy and safety of trifluridine/tipiracil in older and younger patients with metastatic gastric or gastroesophageal junction cancer: subgroup analysis of a randomized phase 3 study (TAGS). Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association, 25:586-597, 2022
12. Miyo M, Kato T, Nakamura Y, Taniguchi H, Takahashi Y, Ishii M, Okita K, Ando K, Yukami H, Mishima S, Yamazaki K, Kotaka M, Watanabe J, Oba K, Aleshin A, Billings PR, Rabinowitz M, Kotani D, Oki E, Takemasa I, Mori M, Yoshino T. DENEB: Development of new criteria for curability after local excision of pathological T1 colorectal cancer using liquid biopsy. Cancer science, 113:1531-1534, 2022
13. Shinmura K, Yamamoto Y, Inaba A, Okumura K, Nishihara K, Kumahara K, Sunakawa H, Furue Y, Ito R, Sato D, Minamide T, Suyama M, Takashima K, Nakajo K, Murano T, Kadota T, Yoda Y, Hori K, Oono Y, Ikematsu H, Yano T. The safety and feasibility of endoscopic submucosal dissection using a flexible three-dimensional endoscope for early gastric cancer and superficial esophageal cancer: A prospective observational study. Journal of gastroenterology and hepatology, 37:749-757, 2022
14. Yamashita H, Kadota T, Minamide T, Sunakawa H, Sato D, Takashima K, Nakajo K, Murano T, Shinmura K, Yoda Y, Ikematsu H, Yano T. Efficacy and safety of second photodynamic therapy for local failure after salvage photodynamic therapy for esophageal cancer. Digestive endoscopy: official journal of the Japan Gastroenterological Endoscopy Society, 34:488-496, 2022
15. Yano T. What is the next critical finding to change our mind in esophagogastroduodenoscopy? Digestive endoscopy: official journal of the Japan Gastroenterological Endoscopy Society, 34:766-768, 2022
16. Ikematsu H, Murano T, Shinmura K. Depth diagnosis of early colorectal cancer: Magnifying chromoendoscopy or image enhanced endoscopy with magnification? Digestive endoscopy: official journal of the Japan Gastroenterological Endoscopy Society, 34:265-273, 2022
17. Mitsui T, Kadota T, Wakabayashi M, Nakajo K, Shinmura K, Sunakawa H, Sato D, Minamide T, Takashima K, Murano T, Yoda Y, Ikematsu H, Yano T. Factors of technical difficulty in conventional and traction-assisted esophageal endoscopic submucosal dissection. Esophagus: official journal of the Japan Esophageal Society, 19:452-459, 2022
18. Ikematsu H, Murano T, Shinmura K. Detection of colorectal lesions during colonoscopy. DEN open, 2:e68, 2022
19. Jinnouchi F, Mori Y, Yoshimoto G, Yamauchi T, Nunomura T, Yurino A, Hayashi M, Yuda J, Shima T, Odawara J, Takashima S, Kamezaki K, Kato K, Miyamoto T, Akashi K, Takenaka K. Incidence of refractory cytomegalovirus infection after allogeneic hematopoietic stem cell transplantation. International journal of hematology, 115:96-106, 2022
20. Tabata R, Sato N, Yamauchi N, Guo YM, Nakamura H, Nagata A, Song-Gi C, Minami Y, Yuda J. Cytomegalovirus reactivation in patients with multiple myeloma administered daratumumab-combination regimens. Annals of hematology, 101:465-467, 2022
21. Mizuno T, Yoshida T, Sunami K, Koyama T, Okita N, Kubo T, Sudo K, Shimoi T, Ueno H, Saito E, Katanoda K, Shibata T, Yonemori K, Okusaka T, Boku N, Ohe Y, Hiroshima Y, Ueno M, Kuboki Y, Doi T, Nakamura K, Kohno T, Yatabe Y, Yamamoto N. Study protocol for NCCH1908 (UPFRONT-trial): a prospective clinical trial to evaluate the feasibility and utility of comprehensive genomic profiling prior to the initial systemic treatment in advanced solid tumour patients. Japanese journal of clinical oncology, 51:1757-1760, 2021
22. Yazaki S, Yoshida T, Kojima Y, Yagishita S, Nakahama H, Okinaka K, Matsushita H, Shiotsuka M, Kobayashi O, Iwata S, Narita Y, Ohba A, Takahashi M, Iwasa S, Kobayashi K, Ohe Y, Yoshida T, Hamada A, Doi T, Yamamoto N. Difference in SARS-CoV-2 Antibody Status Between Patients With Cancer and Health Care Workers During the COVID-19 Pandemic in Japan. JAMA oncology, 7:1141-1148, 2021
23. Shah MA, Bennouna J, Doi T, Shen L, Kato K, Adenis A, Mamon HJ, Moehler M, Fu X, Cho BC, Bordia S, Bhagia P, Shih CS, Desai A, Enzinger P. KEYNOTE-975 study design: a Phase III study of definitive chemoradiotherapy plus pembrolizumab in patients with esophageal carcinoma. Future oncology (London, England), 17:1143-1153, 2021
24. Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, Kojima T, Metges JP, Li Z, Kim SB, Cho BC, Mansoor W, Li SH, Sunpaweravong P, Maqueda MA, Goekkurt E, Hara H, Antunes L, Fountzilas C, Tsuji A, Oliden VC, Liu Q, Shah S, Bhagia P, Kato K. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet (London, England), 398:759-771, 2021
25. Kawai A, Naka N, Shimomura A, Takahashi S, Kitano S, Imura Y, Yonemori K, Nakatani F, Iwata S, Kobayashi E, Outani H, Tamiya H, Naito Y, Yamamoto N, Doi T. Efficacy and safety of TAS-115, a novel oral multi-kinase inhibitor, in osteosarcoma: an expansion cohort of a phase I study. Investigational new drugs, 39:1559-1567, 2021
26. Kimura SI, Tamaki M, Okinaka K, Seo S, Uchida N, Igarashi A, Ozawa Y, Ikegame K, Eto T, Tanaka M, Shiratori S, Nakamae H, Sawa M, Kawakita T, Onizuka M, Fukuda T, Atsuta Y, Kanda Y, Nakasone H. Cytomegalovirus reactivation is associated with an increased risk of late-onset invasive aspergillosis independently of grade II-IV acute graft-versus-host disease in allogeneic hematopoietic stem cell transplantation: JSTCT Transplant Complications Working Group. Annals of hematology, 100:3029-3038, 2021
27. Inoue Y, Okinaka K, Fuji S, Inamoto Y, Uchida N, Toya T, Ikegame K, Eto T, Ozawa Y, Iwato K, Kanda Y, Atsuta Y, Ogata M, Fukuda T. Severe acute graft-versus-host disease increases the incidence of blood stream infection and mortality after allogeneic hematopoietic cell transplantation: Japanese transplant registry study. Bone marrow transplantation, 56:2125-2136, 2021
28. Miura T, Elgersma R, Okizaki A, Inoue MK, Amano K, Mori M, Chitose H, Matsumoto Y, Jager-Wittenaar H, Ottery FD . A Japanese translation, cultural adaptation, and linguistic and content validity confirmation of the Scored Patient-Generated Subjective Global Assessment. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer, 29:7329-7338, 2021
29. Lin CC, Doi T, Muro K, Hou MM, Esaki T, Hara H, Chung HC, Helwig C, Dussault I, Osada M, Kondo S. Bintrafusp Alfa, a Bifunctional Fusion Protein Targeting TGFβ and PD-L1, in Patients with Esophageal Squamous Cell Carcinoma: Results from a Phase 1 Cohort in Asia. Targeted oncology, 16:447-459, 2021
30. Sunami K, Bando H, Yatabe Y, Naito Y, Takahashi H, Tsuchihara K, Toyooka S, Mimori K, Kohsaka S, Uetake H, Kinoshita I, Komine K, Takeda M, Hayashida T, Tamura K, Nishio K, Yamamoto N. Appropriate use of cancer comprehensive genome profiling assay using circulating tumor DNA. Cancer science, 112:3911-3917, 2021
31. Doi T, Yamamoto N, Naito Y, Kuboki Y, Koyama T, Piao Y, Tsujimoto N, Asou H, Inoue K, Kondo S. Merestinib monotherapy or in combination for japanese patients with advanced and/or metastatic cancer: A phase 1 study. Cancer medicine, 10:6579-6589, 2021
32. Shimoi T, Nagai SE, Yoshinami T, Takahashi M, Arioka H, Ishihara M, Kikawa Y, Koizumi K, Kondo N, Sagara Y, Takada M, Takano T, Tsurutani J, Naito Y, Nakamura R, Hattori M, Hara F, Hayashi N, Mizuno T, Miyashita M, Yamashita N, Yamanaka T, Saji S, Iwata H, Toyama T. Correction to: The Japanese Breast Cancer Society Clinical Practice Guidelines for systemic treatment of breast cancer, 2018 edition. Breast cancer (Tokyo, Japan), 28:985-986, 2021
33. Mamishin K, Naito Y, Nomura S, Ogawa G, Niguma K, Baba K, Sakaeda S, Nakajima H, Kusuhara S, Funasaka C, Nakao T, Fukasawa Y, Kondoh C, Harano K, Kogawa T, Matsubara N, Hosono A, Kawasaki T, Mukohara T. Comparison of Treatment Completion Rate Between Conventional and Dose-dense Doxorubicin and Cyclophosphamide (AC) Followed by a Taxane in Patients With Breast Cancer: A Propensity Score-matched Analysis. Anticancer research, 41:6217-6224, 2021
34. Sakaeda S, Naito Y. Circulating Tumor DNA in Oncology. Processes, 9:2198, 2021
35. Naito Y, Kuboki Y, Ikeda M, Harano K, Matsubara N, Toyoizumi S, Mori Y, Hori N, Nagasawa T, Kogawa T. Safety, pharmacokinetics, and preliminary efficacy of the PARP inhibitor talazoparib in Japanese patients with advanced solid tumors: phase 1 study. Investigational new drugs, 39:1568-1576, 2021
36. Kawazoe A, Itahashi K, Yamamoto N, Kotani D, Kuboki Y, Taniguchi H, Harano K, Naito Y, Suzuki M, Fukutani M, Higuchi T, Ikeno T, Wakabayashi M, Sato A, Koyama S, Nishikawa H, Shitara K. TAS-116 (Pimitespib), an Oral HSP90 Inhibitor, in Combination with Nivolumab in Patients with Colorectal Cancer and Other Solid Tumors: An Open-Label, Dose-Finding, and Expansion Phase Ib Trial (EPOC1704). Clinical cancer research: an official journal of the American Association for Cancer Research, 27:6709-6715, 2021
37. Akimoto E, Tokunaga M, Sato R, Yoshida A, Naito Y, Yamashita R, Kinoshita T, Kuwata T. Gastric mesenchymal tumor with smooth muscle differentiation and echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase (EML4-ALK) fusion. Pathology international, 71:707-711, 2021
38. Funasaka C, Naito Y, Kusuhara S, Nakao T, Fukasawa Y, Mamishin K, Komuro A, Okunaka M, Kondoh C, Harano K, Kogawa T, Matsubara N, Hosono A, Kawasaki T, Mukohara T. The efficacy and safety of paclitaxel plus bevacizumab therapy in breast cancer patients with visceral crisis. Breast (Edinburgh, Scotland), 58:50-56, 2021
39. Nishida T, Yoshinaga S, Takahashi T, Naito Y. Recent Progress and Challenges in the Diagnosis and Treatment of Gastrointestinal Stromal Tumors. Cancers, 13:2021
40. Zhu A, Yuan P, Hu N, Li M, Wang W, Wang X, Yue J, Wang J, Luo Y, Ma F, Zhang P, Li Q, Xu B, Cao S, Lippi G, Naito Y, Osman MA, Marta GN, Franceschini G, Orlandi A. Phase II study of apatinib in combination with oral vinorelbine in heavily pretreated HER2-negative metastatic breast cancer and clinical implications of monitoring ctDNA. Cancer biology & medicine, 18:875-887, 2021
41. Doi T, Tajimi M, Mori J, Asou H, Inoue K, Benhadji KA, Naito Y. A phase 1 study of crenigacestat (LY3039478), the Notch inhibitor, in Japanese patients with advanced solid tumors. Investigational new drugs, 39:469-476, 2021
42. Sagara Y, Mori M, Yamamoto S, Eguchi K, Iwatani T, Naito Y, Kogawa T, Tanaka K, Kotani H, Yasojima H, Ozaki Y, Noguchi E, Miyasita M, Kondo N, Niikura N, Toi M, Shien T, Iwata H. Current Status of Advance Care Planning and End-of-life Communication for Patients with Advanced and Metastatic Breast Cancer. The oncologist, 26:e686-e693, 2021
43. Izumi H, Matsumoto S, Liu J, Tanaka K, Mori S, Hayashi K, Kumagai S, Shibata Y, Hayashida T, Watanabe K, Fukuhara T, Ikeda T, Yoh K, Kato T, Nishino K, Nakamura A, Nakachi I, Kuyama S, Furuya N, Sakakibara-Konishi J, Okamoto I, Taima K, Ebi N, Daga H, Yamasaki A, Kodani M, Udagawa H, Kirita K, Zenke Y, Nosaki K, Sugiyama E, Sakai T, Nakai T, Ishii G, Niho S, Ohtsu A, Kobayashi SS, Goto K. The CLIP1-LTK fusion is an oncogenic driver in non-small-cell lung cancer. Nature, 600:319-323, 2021
44. Sakai T, Udagawa H, Kirita K, Nomura S, Itotani R, Tamiya Y, Sugimoto A, Ota T, Naito T, Izumi H, Nosaki K, Ikeda T, Zenke Y, Matsumoto S, Yoh K, Niho S, Nakai T, Ishii G, Goto K. Comparison of the efficiency of endobronchial ultrasound-guided transbronchial needle aspiration using a 22G needle versus 25G needle for the diagnosis of lymph node metastasis in patients with lung cancer: a prospective randomized, crossover study. Translational lung cancer research, 10:3745-3758, 2021
45. Murata S, Sasada S, Usui Y, Sakai T, Kirita K, Ishioka K, Takahashi S, Nakamura M. Omission of Throat Anesthesia Using Jackson's Spray Prior to Bronchoscopy for Preventing Aerosol Generation: A Survey Through Patient Distress Questionnaire. Cureus, 13:e17231, 2021
46. Chida K, Kotani D, Nakamura Y, Kawazoe A, Kuboki Y, Shitara K, Kojima T, Taniguchi H, Watanabe J, Endo I, Yoshino T. Efficacy and safety of trifluridine/tipiracil plus bevacizumab and trifluridine/tipiracil or regorafenib monotherapy for chemorefractory metastatic colorectal cancer: a retrospective study. Therapeutic advances in medical oncology, 13:1.75884E+16, 2021
47. Iida K, Abdelhamid Ahmed AH, Nagatsuma AK, Shibutani T, Yasuda S, Kitamura M, Hattori C, Abe M, Hasegawa J, Iguchi T, Karibe T, Nakada T, Inaki K, Kamei R, Abe Y, Nomura T, Andersen JL, Santagata S, Hemming ML, George S, Doi T, Ochiai A, Demetri GD, Agatsuma T. Identification and Therapeutic Targeting of GPR20, Selectively Expressed in Gastrointestinal Stromal Tumors, with DS-6157a, a First-in-Class Antibody-Drug Conjugate. Cancer discovery, 11:1508-1523, 2021
48. Oki E, Watanabe J, Sato T, Kagawa Y, Kuboki Y, Ikeda M, Ueno H, Kato T, Kusumoto T, Masuishi T, Yamaguchi K, Kanazawa A, Nishina T, Uetake H, Yamanaka T, Yoshino T. Impact of the 12-gene recurrence score assay on deciding adjuvant chemotherapy for stage II and IIIA/B colon cancer: the SUNRISE-DI study. ESMO open, 6:100146, 2021
49. Chida K, Kawazoe A, Kawazu M, Suzuki T, Nakamura Y, Nakatsura T, Kuwata T, Ueno T, Kuboki Y, Kotani D, Kojima T, Taniguchi H, Mano H, Ikeda M, Shitara K, Endo I, Yoshino T. A Low Tumor Mutational Burden and PTEN Mutations Are Predictors of a Negative Response to PD-1 Blockade in MSI-H/dMMR Gastrointestinal Tumors. Clinical cancer research: an official journal of the American Association for Cancer Research, 27:3714-3724, 2021
50. Taniguchi H, Nakamura Y, Kotani D, Yukami H, Mishima S, Sawada K, Shirasu H, Ebi H, Yamanaka T, Aleshin A, Billings PR, Rabinowitz M, Oki E, Takemasa I, Kato T, Mori M, Yoshino T. CIRCULATE-Japan: Circulating tumor DNA-guided adaptive platform trials to refine adjuvant therapy for colorectal cancer. Cancer science, 112:2915-2920, 2021
51. Mishima S, Shitara K. Trastuzumab deruxtecan for the treatment of HER2-positive gastric cancer. Expert opinion on biological therapy, 21:825-830, 2021
52. Kato T, Kagawa Y, Kuboki Y, Gamoh M, Komatsu Y, Yasui H, Satake H, Oki E, Tanioka H, Kotaka M, Makiyama A, Denda T, Goto M, Yoshino T, Yamazaki K, Soeda J, Shibuya K, Iwata M, Oba K, Yamaguchi K. Safety and efficacy of panitumumab in combination with trifluridine/tipiracil for pre-treated patients with unresectable, metastatic colorectal cancer with wild-type RAS: The phase 1/2 APOLLON study. International journal of clinical oncology, 26:1238-1247, 2021
53. Mansoor W, Arkenau HT, Alsina M, Shitara K, Thuss-Patience P, Cuffe S, Dvorkin M, Park D, Ando T, Van Den Eynde M, Beretta GD, Zaniboni A, Doi T, Tabernero J, Ilson DH, Makris L, Benhadji KA, Van Cutsem E. Trifluridine/tipiracil in patients with metastatic gastroesophageal junction cancer: a subgroup analysis from the phase 3 TAGS study. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association, 24:970-977, 2021
54. Curigliano G, Gelderblom H, Mach N, Doi T, Tai D, Forde PM, Sarantopoulos J, Bedard PL, Lin CC, Hodi FS, Wilgenhof S, Santoro A, Sabatos-Peyton CA, Longmire TA, Xyrafas A, Sun H, Gutzwiller S, Manenti L, Naing A. Phase I/Ib Clinical Trial of Sabatolimab, an Anti-TIM-3 Antibody, Alone and in Combination with Spartalizumab, an Anti-PD-1 Antibody, in Advanced Solid Tumors. Clinical cancer research: an official journal of the American Association for Cancer Research, 27:3620-3629, 2021
55. Takushima Y, Igarashi A, Yoshihara H, Shitara K, Doi T. Cost-effectiveness of trifluridine/tipiracil against nivolumab for heavily pretreated metastatic gastric cancer in Japan. Japanese journal of clinical oncology, 51:1383-1390, 2021
56. Piha-Paul SA, Geva R, Tan TJ, Lim DW, Hierro C, Doi T, Rahma O, Lesokhin A, Luke JJ, Otero J, Nardi L, Singh A, Xyrafas A, Chen X, Mataraza J, Bedard PL. First-in-human phase I/Ib open-label dose-escalation study of GWN323 (anti-GITR) as a single agent and in combination with spartalizumab (anti-PD-1) in patients with advanced solid tumors and lymphomas. Journal for immunotherapy of cancer, 9:2021
57. Jogo T, Nakamura Y, Shitara K, Bando H, Yasui H, Esaki T, Terazawa T, Satoh T, Shinozaki E, Nishina T, Sunakawa Y, Komatsu Y, Hara H, Oki E, Matsuhashi N, Ohta T, Kato T, Ohtsubo K, Kawakami T, Okano N, Yamamoto Y, Yamada T, Tsuji A, Odegaard JI, Taniguchi H, Doi T, Fujii S, Yoshino T. Circulating Tumor DNA Analysis Detects FGFR2 Amplification and Concurrent Genomic Alterations Associated with FGFR Inhibitor Efficacy in Advanced Gastric Cancer. Clinical cancer research: an official journal of the American Association for Cancer Research, 27:5619-5627, 2021
58. Okuyama H, Kagawa Y, Masuishi T, Mishima S, Shirasu H, Ando K, Yuki S, Muro K, Yoshino T, Yamazaki K, Oki E, Komatsu Y, Tsuji A. Infusion-related reaction to ramucirumab plus FOLFIRI in patients with advanced colorectal cancer. International journal of clinical oncology, 26:2025-2028, 2021
59. Maeda Y, Wada H, Sugiyama D, Saito T, Irie T, Itahashi K, Minoura K, Suzuki S, Kojima T, Kakimi K, Nakajima J, Funakoshi T, Iida S, Oka M, Shimamura T, Doi T, Doki Y, Nakayama E, Ueda R, Nishikawa H. Depletion of central memory CD8+ T cells might impede the antitumor therapeutic effect of Mogamulizumab. Nature communications, 12:7280, 2021
60. Hori K, Ikematsu H, Yamamoto Y, Matsuzaki H, Takeshita N, Shinmura K, Yoda Y, Kiuchi T, Takemoto S, Yokota H, Yano T. Detecting colon polyps in endoscopic images using artificial intelligence constructed with automated collection of annotated images from an endoscopy reporting system. Digestive endoscopy: official journal of the Japan Gastroenterological Endoscopy Society, 2021
61. Hashimoto Y, Kumahara K, Ueda Y, Tahara M. Cholangioscopic finding of severe hemorrhagic cholangitis associated with immune-related adverse events. Gastrointestinal endoscopy, 94:859-860, 2021
62. Yuda J, Yamauchi N, Kuzume A, Guo YM, Sato N, Minami Y. Molecular remission after combination therapy with blinatumomab and ponatinib with relapsed/refractory Philadelphia chromosome-positive acute lymphocytic leukemia: two case reports. Journal of medical case reports, 15:164, 2021
63. H Nakamura, Y Minami, SG Chi, A Nagata, S Uchiyama, N Yamauchi. Emerging Mitochondria-Associated Molecular Target Therapies for Acute Myeloid Leukemia. Arch Clin Biomed Res, 5:419-424, 2021
64. Harding JJ, Moreno V, Bang YJ, Hong MH, Patnaik A, Trigo J, Szpurka AM, Yamamoto N, Doi T, Fu S, Calderon B, Velez de Mendizabal N, Calvo E, Yu D, Gandhi L, Liu ZT, Galvao VR, Leow CC, de Miguel MJ. Blocking TIM-3 in Treatment-refractory Advanced Solid Tumors: A Phase Ia/b Study of LY3321367 with or without an Anti-PD-L1 Antibody. Clinical cancer research: an official journal of the American Association for Cancer Research, 27:2168-2178, 2021
65. Hellmann MD, Bivi N, Calderon B, Shimizu T, Delafontaine B, Liu ZT, Szpurka AM, Copeland V, Hodi FS, Rottey S, Aftimos P, Piao Y, Gandhi L, Galvao VR, Leow CC, Doi T. Safety and Immunogenicity of LY3415244, a Bispecific Antibody Against TIM-3 and PD-L1, in Patients With Advanced Solid Tumors. Clinical cancer research: an official journal of the American Association for Cancer Research, 27:2773-2781, 2021
66. Aoki H, Ueha S, Shichino S, Ogiwara H, Shitara K, Shimomura M, Suzuki T, Nakatsura T, Yamashita M, Kitano S, Kuroda S, Wakabayashi M, Kurachi M, Ito S, Doi T, Matsushima K. Transient Depletion of CD4(+) Cells Induces Remodeling of the TCR Repertoire in Gastrointestinal Cancer. Cancer immunology research, 9:624-636, 2021
67. Sakai T, Udagawa H, Matsumoto S, Yoh K, Nosaki K, Ikeda T, Zenke Y, Kirita K, Niho S, Akimoto T, Goto K, Ishii G. Morphological, immune and genetic features in biopsy sample associated with the efficacy of pembrolizumab in patients with non-squamous non-small cell lung cancer. Journal of cancer research and clinical oncology, 147:1227-1237, 2021
68. Mukohara T, Hosono A, Mimaki S, Nakayama A, Kusuhara S, Funasaka C, Nakao T, Fukasawa Y, Kondoh C, Harano K, Naito Y, Matsubara N, Tsuchihara K, Kuwata T. Effects of Ado-Trastuzumab Emtansine and Fam-Trastuzumab Deruxtecan on Metastatic Breast Cancer Harboring HER2 Amplification and the L755S Mutation. The oncologist, 26:635-639, 2021
69. Terao T, Yuda J, Yamauchi N, Guo YM, Shimada K, Sugano M, Ishii G, Minami Y. Brentuximab vedotin maintenance after autologous stem cell transplantation for refractory gray zone lymphoma with long-term remission. Molecular and clinical oncology, 14:125, 2021
70. Kosugi K, Nishiguchi Y, Miura T, Fujisawa D, Kawaguchi T, Izumi K, Takehana J, Uehara Y, Usui Y, Terada T, Inoue Y, Natsume M, Yajima MY, Watanabe YS, Okizaki A, Matsushima E, Matsumoto Y. Association Between Loneliness and the Frequency of Using Online Peer Support Groups Among Cancer Patients With Minor Children: A Cross-Sectional Web-Based Study. Journal of pain and symptom management, 61:955-962, 2021
71. Nakajo K, Yoda Y, Kadota T, Murano T, Shinmura K, Ikematsu H, Akimoto T, Yano T. Radial incision and cutting for dilation before endoscopic submucosal dissection in patients with esophageal cancer on the distal side of severe benign esophageal strictures. Diseases of the esophagus: official journal of the International Society for Diseases of the Esophagus, 34:2021
72. Ito R, Kadota T, Murano T, Yoda Y, Hori K, Minamide T, Sato D, Yamamoto Y, Takashima K, Shinmura K, Ikematsu H, Yano T. Clinical features and risk factors of gastric cancer detected by esophagogastroduodenoscopy in esophageal cancer patients. Esophagus: official journal of the Japan Esophageal Society, 18:621-628, 2021
