Annual Report 2021
Department of Genetic Medicine and Services
Takeshi Kuwata, Kazuya Tsuchihara, Toru Mukohara, Kiwamu Akagi, Akiko Nakayama, Sachiyo Mimaki, Yumie Hiraoka, Kaori Kimura, Manami Matsukawa, Chikako Tomozawa, Kenichi Harano, Shingo Matsumoto, Kyoko Toju, Nobuyuki Nakamura, Tsuyoshi Uemoto, Yoshiko Onuma
Introduction
The Department of Genetic Medicine and Services was newly established in May 2016, to handle genetic as well as genomic testing and related issues, including genetic counseling, conducted in the National Cancer Center Hospital East (NCCHE).
The Team and What We Do
The aim of the Department of Genetic Medicine and Services is to deal with the following subjects:
1) Genetic counseling
2) Genetic/genomic testings
3) Ethical issues related to genetic/genomic medicine
4) Educating people on genetic/genomic medicine
5) Regulating the storage and usage of genetic/genomic information
6) Other matters related to genetic/genomic medicine
Accordingly, members of the department with various specialties were assembled, including medical doctors, nurses, genetic counselors and medical technicians from different clinical departments or research laboratories.
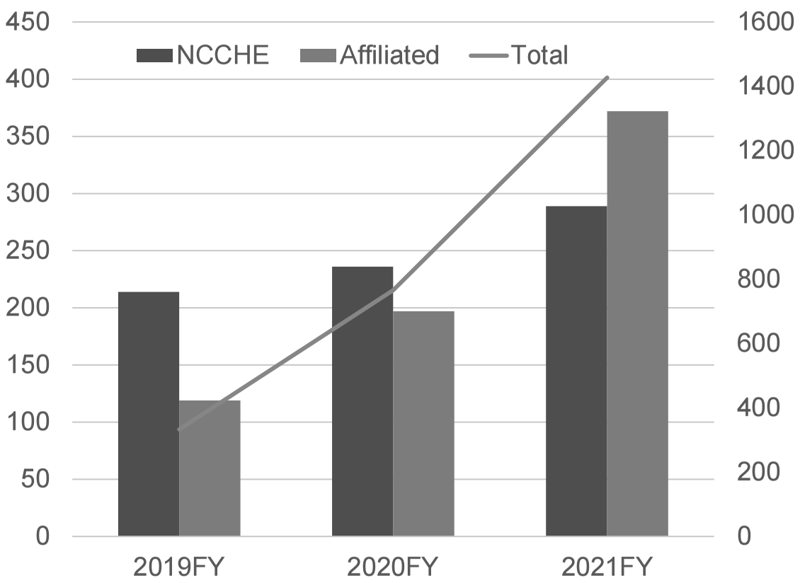
To conduct precision oncology, our hospital is designated as a Cancer Genome Medical Core Hospital in Japan and cooperates with 9 affiliated hospitals, especially for providing cancer genome profiling (CGP) tests. Under the national health care services, by March 2022, we performed 285 CGP tests and conducted expert panels for 289 patients, including 372 patients in affiliated hospitals (Figure 1).
Figure 1. Number of CGP cases reviewed in expert panels

The Outpatient Genetic Counseling Clinic provides genetic counseling and genetic testing for cancer patients and their relatives with a familial history and/or specific features suspected for familial cancers. From April 2021 to March 2022, 118 new clients visited the clinic, and a total of 222 genetic counseling sessions were held and 58 genetic testing sessions were provided. Consequently, by March 2022, a total of 320 clients had visited the clinic (Figures 2 and 3). The department also participated in an outpatient clinic specializing in Hereditary Breast and Ovary Cancer (HBOC) syndrome established by the Department of Breast and Medical Oncology.
Figure 2. Number of genetic counseling sessions provided
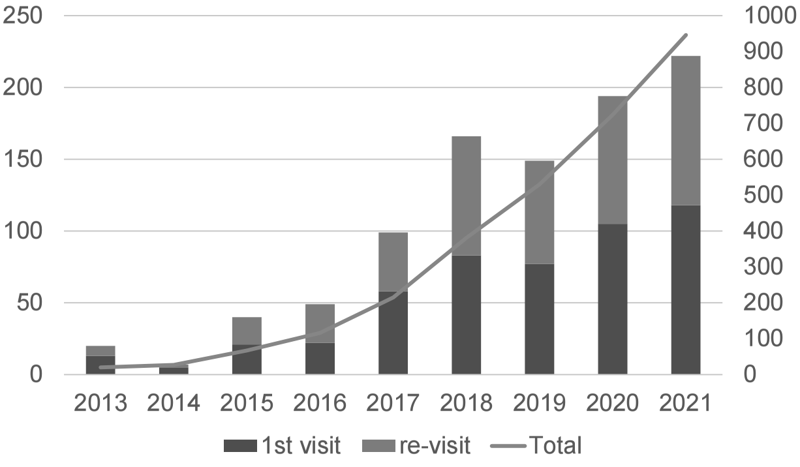

Figure 3. Breakdown of genetic counseling session 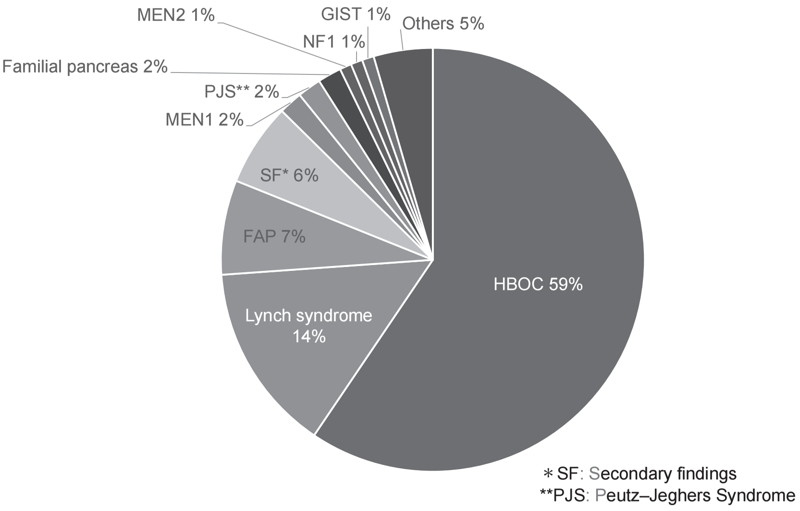

Research activities
Our Outpatient Genetic Counseling Clinic is participating in the NCC Research and Development Fund program (25-A-1) and provides genetic testing.
Clinical trials
Our Outpatient Genetic Counseling Clinic supports and provides genetic counseling for patients willing to participate in clinical trials where genetic tests are required.
Education
We educated medical doctors wishing to become board-certified geneticists by attending the Outpatient Genetic Counseling Clinic. We have also provided intramural educational seminars for medical doctors and paramedical personnel to familiarize them with genetic and genomic medicine and provide precision medicine for all cancer patients and their families visiting our hospital. As a Cancer Genome Medical Core Hospital, we also provide seminars and lectures for educating doctors as well as paramedical personnel working at affiliated hospitals.
Future Prospects
We will continue to provide genetic counseling and genetic testing for possible familial cancer patients/families. For precision oncology, we will conduct CGP tests under the national health care system and participate in several clinical trials using new technologies including liquid biopsy and whole genome/exome sequences. We will also continue to provide education programs for medical doctors and paramedics working in our hospital as well as affiliated hospitals. Our current aim is to establish a genomic testing pipeline from research to clinic to accelerate the development of medical and diagnostic devices for genome medicine.
List of papers published in 2021
Journal
1. Kumagai S, Koyama S, Itahashi K, Tanegashima T, Lin YT, Togashi Y, Kamada T, Irie T, Okumura G, Kono H, Ito D, Fujii R, Watanabe S, Sai A, Fukuoka S, Sugiyama E, Watanabe G, Owari T, Nishinakamura H, Sugiyama D, Maeda Y, Kawazoe A, Yukami H, Chida K, Ohara Y, Yoshida T, Shinno Y, Takeyasu Y, Shirasawa M, Nakama K, Aokage K, Suzuki J, Ishii G, Kuwata T, Sakamoto N, Kawazu M, Ueno T, Mori T, Yamazaki N, Tsuboi M, Yatabe Y, Kinoshita T, Doi T, Shitara K, Mano H, Nishikawa H. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer cell, 40:201-218.e9, 2022
2. Ozeki R, Iihara H, Shimokawa M, Hashimoto H, Abe M, Mukohara T, Bando H, Hayashi T, Kawazoe H, Komoda M, Yanai Takahashi T, Saito M. Study protocol for a double-blind, comparative, randomised Japanese trial of triplet standard antiemetic therapies with or without 5 mg olanzapine to prevent chemotherapy-induced nausea and vomiting for patients with breast cancer treated with an anthracycline/cyclophosphamide regimen (JTOP-B). BMJ open, 12:e058755, 2022
3. Masuda H, Harano K, Miura S, Wang Y, Hirota Y, Harada O, Jolly MK, Matsunaga Y, Lim B, Wood AL, Parinyanitikul N, Jin Lee H, Gong G, George JT, Levine H, Lee J, Wang X, Lucci A, Rao A, Schweitzer BL, Lawrence OR, Seitz RS, Morris SW, Hout DR, Nakamura S, Krishnamurthy S, Ueno NT. Changes in Triple-Negative Breast Cancer Molecular Subtypes in Patients Without Pathologic Complete Response After Neoadjuvant Systemic Chemotherapy. JCO precision oncology, 6:e2000368, 2022
4. Nakajima H, Harano K, Nakai T, Kusuhara S, Nakao T, Funasaka C, Kondoh C, Matsubara N, Naito Y, Hosono A, Mitsunaga S, Ishii G, Mukohara T. Impacts of clinicopathological factors on efficacy of trastuzumab deruxtecan in patients with HER2-positive metastatic breast cancer. Breast (Edinburgh, Scotland), 61:136-144, 2022
5. Ikematsu H, Ishihara M, Okawa S, Minamide T, Mitsui T, Kuwata T, Ito M, Kinoshita T, Fujita T, Yano T, Omori T, Ozawa S, Murakoshi D, Irisawa K, Ochiai A. Photoacoustic imaging of fresh human surgically and endoscopically resected gastrointestinal specimens. DEN open, 2:e28, 2022
6. Chida K, Kawazoe A, Suzuki T, Kawazu M, Ueno T, Takenouchi K, Nakamura Y, Kuboki Y, Kotani D, Kojima T, Bando H, Mishima S, Kuwata T, Sakamoto N, Watanabe J, Mano H, Ikeda M, Shitara K, Endo I, Nakatsura T, Yoshino T. Transcriptomic Profiling of MSI-H/dMMR Gastrointestinal Tumors to Identify Determinants of Responsiveness to Anti-PD-1 Therapy. Clinical cancer research: an official journal of the American Association for Cancer Research, 28:2110-2117, 2022
7. Yanagihara K, Iino Y, Yokozaki H, Kubo T, Oda T, Kubo T, Komatsu M, Sasaki H, Ichikawa H, Kuwata T, Seyama T, Ochiai A. A Comparative Study of Patient-Derived Tumor Models of Pancreatic Ductal Adenocarcinoma Involving Orthotopic Implantation. Pathobiology: journal of immunopathology, molecular and cellular biology, 1-11, 2022
8. Ishizu K, Hashimoto T, Naka T, Yatabe Y, Kojima M, Kuwata T, Nonaka S, Oda I, Esaki M, Kudo M, Gotohda N, Yoshida T, Yoshikawa T, Sekine S. APC mutations are common in adenomas but infrequent in adenocarcinomas of the non-ampullary duodenum. Journal of gastroenterology, 56:988-998, 2021
9. Mamishin K, Naito Y, Nomura S, Ogawa G, Niguma K, Baba K, Sakaeda S, Nakajima H, Kusuhara S, Funasaka C, Nakao T, Fukasawa Y, Kondoh C, Harano K, Kogawa T, Matsubara N, Hosono A, Kawasaki T, Mukohara T. Comparison of Treatment Completion Rate Between Conventional and Dose-dense Doxorubicin and Cyclophosphamide (AC) Followed by a Taxane in Patients With Breast Cancer: A Propensity Score-matched Analysis. Anticancer research, 41:6217-6224, 2021
10. El Bairi K, Haynes HR, Blackley E, Fineberg S, Shear J, Turner S, de Freitas JR, Sur D, Amendola LC, Gharib M, Kallala A, Arun I, Azmoudeh-Ardalan F, Fujimoto L, Sua LF, Liu SW, Lien HC, Kirtani P, Balancin M, El Attar H, Guleria P, Yang W, Shash E, Chen IC, Bautista V, Do Prado Moura JF, Rapoport BL, Castaneda C, Spengler E, Acosta-Haab G, Frahm I, Sanchez J, Castillo M, Bouchmaa N, Md Zin RR, Shui R, Onyuma T, Yang W, Husain Z, Willard-Gallo K, Coosemans A, Perez EA, Provenzano E, Ericsson PG, Richardet E, Mehrotra R, Sarancone S, Ehinger A, Rimm DL, Bartlett JMS, Viale G, Denkert C, Hida AI, Sotiriou C, Loibl S, Hewitt SM, Badve S, Symmans WF, Kim RS, Pruneri G, Goel S, Francis PA, Inurrigarro G, Yamaguchi R, Garcia-Rivello H, Horlings H, Afqir S, Salgado R, Adams S, Kok M, Dieci MV, Michiels S, Demaria S, Loi S. The tale of TILs in breast cancer: A report from The International Immuno-Oncology Biomarker Working Group. NPJ breast cancer, 7:150, 2021
11. Naito Y, Kuboki Y, Ikeda M, Harano K, Matsubara N, Toyoizumi S, Mori Y, Hori N, Nagasawa T, Kogawa T. Safety, pharmacokinetics, and preliminary efficacy of the PARP inhibitor talazoparib in Japanese patients with advanced solid tumors: phase 1 study. Investigational new drugs, 39:1568-1576, 2021
12. Kawazoe A, Itahashi K, Yamamoto N, Kotani D, Kuboki Y, Taniguchi H, Harano K, Naito Y, Suzuki M, Fukutani M, Higuchi T, Ikeno T, Wakabayashi M, Sato A, Koyama S, Nishikawa H, Shitara K. TAS-116 (Pimitespib), an Oral HSP90 Inhibitor, in Combination with Nivolumab in Patients with Colorectal Cancer and Other Solid Tumors: An Open-Label, Dose-Finding, and Expansion Phase Ib Trial (EPOC1704). Clinical cancer research: an official journal of the American Association for Cancer Research, 27:6709-6715, 2021
13. Colombo N, Dubot C, Lorusso D, Caceres MV, Hasegawa K, Shapira-Frommer R, Tewari KS, Salman P, Hoyos Usta E, Yañez E, Gümüş M, Olivera Hurtado de Mendoza M, Samouë:lian V, Castonguay V, Arkhipov A, Toker S, Li K, Keefe SM, Monk BJ . Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer. The New England journal of medicine, 385:1856-1867, 2021
14. Funasaka C, Naito Y, Kusuhara S, Nakao T, Fukasawa Y, Mamishin K, Komuro A, Okunaka M, Kondoh C, Harano K, Kogawa T, Matsubara N, Hosono A, Kawasaki T, Mukohara T. The efficacy and safety of paclitaxel plus bevacizumab therapy in breast cancer patients with visceral crisis. Breast (Edinburgh, Scotland), 58:50-56, 2021
15. Oiwa H, Aokage K, Suzuki A, Sato K, Kuroe T, Mimaki S, Tane K, Miyoshi T, Samejima J, Tsuchihara K, Goto K, Funai K, Tsuboi M, Nakai T, Ishii G. Clinicopathological, gene expression and genetic features of stage I lung adenocarcinoma with necrosis. Lung cancer (Amsterdam, Netherlands), 159:74-83, 2021
16. Ishii T, Suzuki A, Kuwata T, Hisamitsu S, Hashimoto H, Ohara Y, Yanagihara K, Mitsunaga S, Yoshino T, Kinoshita T, Ochiai A, Shitara K, Ishii G. Drug-exposed cancer-associated fibroblasts facilitate gastric cancer cell progression following chemotherapy. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association, 24:810-822, 2021
17. Chida K, Kawazoe A, Kawazu M, Suzuki T, Nakamura Y, Nakatsura T, Kuwata T, Ueno T, Kuboki Y, Kotani D, Kojima T, Taniguchi H, Mano H, Ikeda M, Shitara K, Endo I, Yoshino T. A Low Tumor Mutational Burden and PTEN Mutations Are Predictors of a Negative Response to PD-1 Blockade in MSI-H/dMMR Gastrointestinal Tumors. Clinical cancer research: an official journal of the American Association for Cancer Research, 27:3714-3724, 2021
18. Hatanaka Y, Kuwata T, Morii E, Kanai Y, Ichikawa H, Kubo T, Hatanaka KC, Sakai K, Nishio K, Fujii S, Okamoto W, Yoshino T, Ochiai A, Oda Y. The Japanese Society of Pathology Practical Guidelines on the handling of pathological tissue samples for cancer genomic medicine. Pathology international, 71:725-740, 2021
19. Sato D, Takamatsu T, Umezawa M, Kitagawa Y, Maeda K, Hosokawa N, Okubo K, Kamimura M, Kadota T, Akimoto T, Kinoshita T, Yano T, Kuwata T, Ikematsu H, Takemura H, Yokota H, Soga K. Author Correction: Distinction of surgically resected gastrointestinal stromal tumor by near-infrared hyperspectral imaging. Scientific reports, 11:19030, 2021
20. Ishioka K, Yasuda H, Hamamoto J, Terai H, Emoto K, Kim TJ, Hirose S, Kamatani T, Mimaki S, Arai D, Ohgino K, Tani T, Masuzawa K, Manabe T, Shinozaki T, Mitsuishi A, Ebisudani T, Fukushima T, Ozaki M, Ikemura S, Kawada I, Naoki K, Nakamura M, Ohtsuka T, Asamura H, Tsuchihara K, Hayashi Y, Hegab AE, Kobayashi SS, Kohno T, Watanabe H, Ornitz DM, Betsuyaku T, Soejima K, Fukunaga K. Upregulation of FGF9 in Lung Adenocarcinoma Transdifferentiation to Small Cell Lung Cancer. Cancer research, 81:3916-3929, 2021
21. Mukohara T, Hosono A, Mimaki S, Nakayama A, Kusuhara S, Funasaka C, Nakao T, Fukasawa Y, Kondoh C, Harano K, Naito Y, Matsubara N, Tsuchihara K, Kuwata T. Effects of Ado-Trastuzumab Emtansine and Fam-Trastuzumab Deruxtecan on Metastatic Breast Cancer Harboring HER2 Amplification and the L755S Mutation. The oncologist, 26:635-639, 2021
22. Takamatsu T, Kitagawa Y, Akimoto K, Iwanami R, Endo Y, Takashima K, Okubo K, Umezawa M, Kuwata T, Sato D, Kadota T, Mitsui T, Ikematsu H, Yokota H, Soga K, Takemura H. Over 1000 nm Near-Infrared Multispectral Imaging System for Laparoscopic In Vivo Imaging. Sensors (Basel, Switzerland), 21:2021
