Annual Report 2021
Department of Clinical Laboratories
Nobuo Kuninaka, Hiroshi Yamakawa, Masahiro Karibe, Yuko Adegawa, Momoko Iguchi, Masaki Takeda, Kenta Akie, Nobuyuki Nakamura, Mizuho Fujima, Michiteru Yamagishi, Atsuko Uno, Yasuharu Hashimoto, Michiko Iida, Saki Nakamura, Yuki Takahashi, Tomofumi Tan, Keisei Nakamura, Akira Miyaura, Mika Sasanuma, Rana Goto, Momoka Sakayori, Takuya Hanazawa, Ayaka Takahashi, Keiko Nakai, Ayumi Nakanishi, Misato Nojiri, Risako Ueda, Hitomi Nakamura, Sayuri Shibayama, Tomomi Wachi, Go Sato, Mari Hisano, Mitsunori Tajima, Yui Jinbo, Masayuki Ito, Yuki Takano, Riko Kawarai, Kazuma Takada, Mika Narikiyo, Takuya Aiba, Ayumi Setsuda, Takaki Kobayashi, Chiharu Eda, Kentaro Yamada, Masayuki Sukegawa, Shota Oishi, Yuichiro Yazaki, Yuma Furuya, Masahiro Inoue, Hikari Sekiguchi, Jun Ikoma, Aiko Kimura, Yumiko Ito, Ayumi Iwaya, Aya Koike, Tomoko Ikeda, Tomoe Katakura, Kaori Toyama, Ayaka Ohashi, Yoriko Furusawa, Izumi Suzuki, Haruka Nozaki, Megumi Yamaguchi, Mariko Kinoshita, Yasuko Nakazeko, Chiho Furuya, Noriko Sato, Kyoko Kuwayama, Mana Shimamura, Kanako Nakayama
Introduction
In 2021, two years had passed since the department became independent from the Department of Pathology and Clinical Laboratories, and we were proceeding with stable operations.
The quality control and technical capabilities of the Department of Clinical Laboratories are based on the international standard ISO 15189, and we have created a system for carrying out all laboratory work, including laboratory tests, physiological function tests, clinical trials, and pathology research, in accordance with international standards.
The Team and What We Do
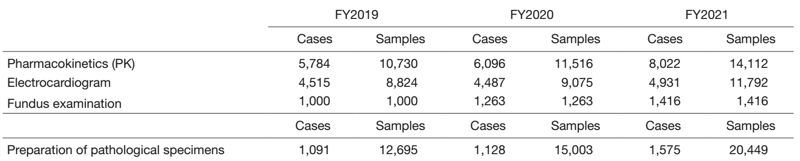
The total number of clinical examinations was 4,353,114, an increase of 6% from the previous year, and increasing year by year (Table 1). Compared to the previous year, the number of clinical trial examinations was 23% for sample processing (PK), 29% for electrocardiogram examinations, 12% for fundus examinations, and 36% for pathological specimen preparation, showing an increasing trend in all fields (Table 2).
Table 1. Number of clinical tests performed in FY2021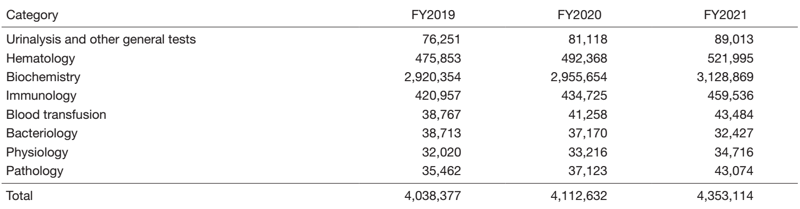

Table 2. Number of clinical trial tests performed in FY2021

Research activities
-
Introduction of LBC for fine needle aspiration cytology for the
head and neck region
→ In FY2021, we started single LBC tests, except for suspected malignant lymphoma. -
Introduction of rapid testing by UFP staining in the EUS-FNA
region and introduction of LBC
→ In 2021, DQ was changed to UFP, and UFP staining was introduced for rapid testing. -
Introduction of rapid LBC for intraoperative rapid cytological
examinations (gastric cancer area)
→ There were some cases that could not be diagnosed up to Class V by the drag glass method alone, but about three cases in half a year were diagnosed as Class V using rapid LBC specimens. Rapid LBC specimens are specimens with the same quality as LBC specimens produced by the manufacturer’s recommended method, which enables the same diagnoses to be made. -
Investigation of immunostaining conditions for cytological
specimens (especially the head and neck region)
→ Basically, there is no problem under the same conditions as for histological specimens. Clinically, immunostaining with cytological specimens is required for cases in which tissue biopsy is difficult.
Education
We have established an education and training system based on the ISO15189 certification requirements of the Japan Accreditation Board (JAB). Study groups, workshops, and lectures are held once a month to improve the academic knowledge, skills, and ethics of faculty and staff. In addition, in our laboratory, we have strived to improve the skills of our staff by designing educational programs tailored to each skill level. In striving to improve skills, proficiency is determined by two methods: self-evaluation and evaluation by an evaluator using an operation comprehension check sheet.
Laboratory staff are fully aware of the role of the National Center, and are striving to improve their skills and knowledge. Through the three pillars of daily work, education, and research, we will continue to raise the standards of clinical laboratories.
We are working to develop the next generation, and also actively accept clinical trainees. In 2021, we will accept seven trainees from Tsukuba International University, one trainee from Daito Bunka University, and two trainees from Kyorin University.
Future Prospects
Clinical laboratories form the basis of not only daily clinical practice but also medical research. As an ISO15189 accredited pathological diagnosis and clinical testing facility, Hospital East conducts medical care and clinical research. With the goal of 2022, preparations have begun with the aim of obtaining CAP (College of American Pathologists) certification. In the future, we will strengthen cooperation with other departments of Hospital East and the Advanced Medical Development Center and actively participate in the development of new drugs and devices. We will contribute to the implementation of Hospital East's vision of “providing the world's best cancer care and creating new world-class cancer care.”
