Annual Report 2021
Department of Medical Oncology
Kan Yonemori, Tatsunori Shimoi, Kazuki Sudo, Tadaaki Nishikawa, Yuki Kojima, Kasumi Yamamoto, Shosuke Kita, Ayumi Saito, Motoko Arakaki, Hitomi Sumiyoshi-Okuma, Chiharu Mizoguchi, Shu Yazaki, Momoko Tokura, Yohei Chiba, Yasuhiro Fujiwara, Emi Noguchi
Introduction
The Department of Medical Oncology provides the most effective treatments by the use of chemotherapy, and works on the establishment of new standards of care for adult malignancies including breast cancer, gynecologic cancer, Soft-tissue sarcoma and malignant bone tumor, extra-gonadal germ cell tumor, cancer of unknown primary site and other rare types of solid tumors.
We envision becoming a leading medical oncology department, which makes a difference in cancer care in Japan and in the world. Our mission is to provide patient-centered, state-of-the-art medical care to cancer patients, to develop new effective cancer treatments through clinical and translational research, and to nurture medical oncologists. An evidence-based, research-oriented and multidisciplinary approach is the core value of our practice.
The Team and What We Do
The Department of Oncology conducts clinical, research and educational activities with a focus on pharmacotherapy for breast, uterine, ovarian, and malignant soft tissue tumors (e.g. sarcomas), germ cell tumors, cancers of unknown primary site, urological cancers and other rare malignancies (Table 1).
Table 1. 1st Visiting Patients to the Department of
Medical Oncology (Apr.2020 - Mar, 2021)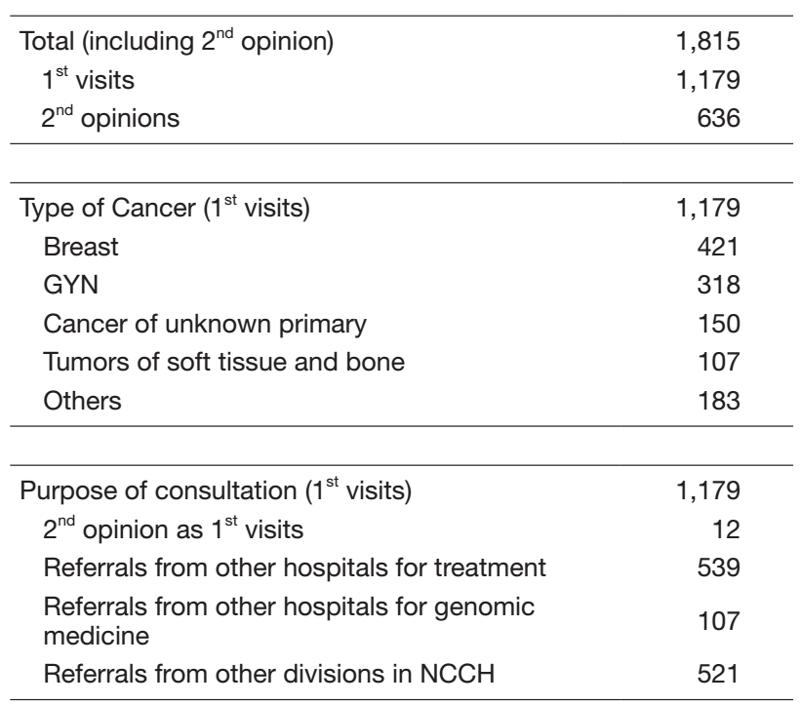

The five basic missions are as follows
- Contribute to the development of new cancer therapies domestically and globally by planning, supervising or participating in clinical trials.
- Promote translational research and strengthen cooperation with other departments, other professions and other research centers.
- Lead the world in the development of treatments for breast cancer, gynecological cancer and sarcoma, and in the early clinical development of anti-cancer drugs.
- pharmacotherapy and breast cancer treatment (medical specialists).
- Understand the physical, mental and social suffering of cancer patients and aim for holistic medical care.
In addition to providing the latest evidence-based pharmacotherapy, the department focuses on research activities aimed at building new evidence, focusing on the social environment surrounding cancer care, and developing treatments for rare cancers.
Research activities
Our research interest extends across a wide range of topics related to treatment and clinical program development. Many of our research programs are secured by public and consignment research grants. In 2021, we conducted many research programs as a primary investigator and participated as a co-investigator in additional research programs secured by competitive public research funds.
We published 43 international manuscripts, focusing on early-phase anti-cancer drug development, molecular imaging, drug efficacy studies using patient derived xenografts, translational research, novel chemotherapy against sarcoma and ovarian cancer, novel biomarkers to predict efficacy and adverse events of anti-cancer drugs and other basic research. We value cancer survivorship as a research theme in order to develop a comprehensive patient-centered care program.
Clinical trials
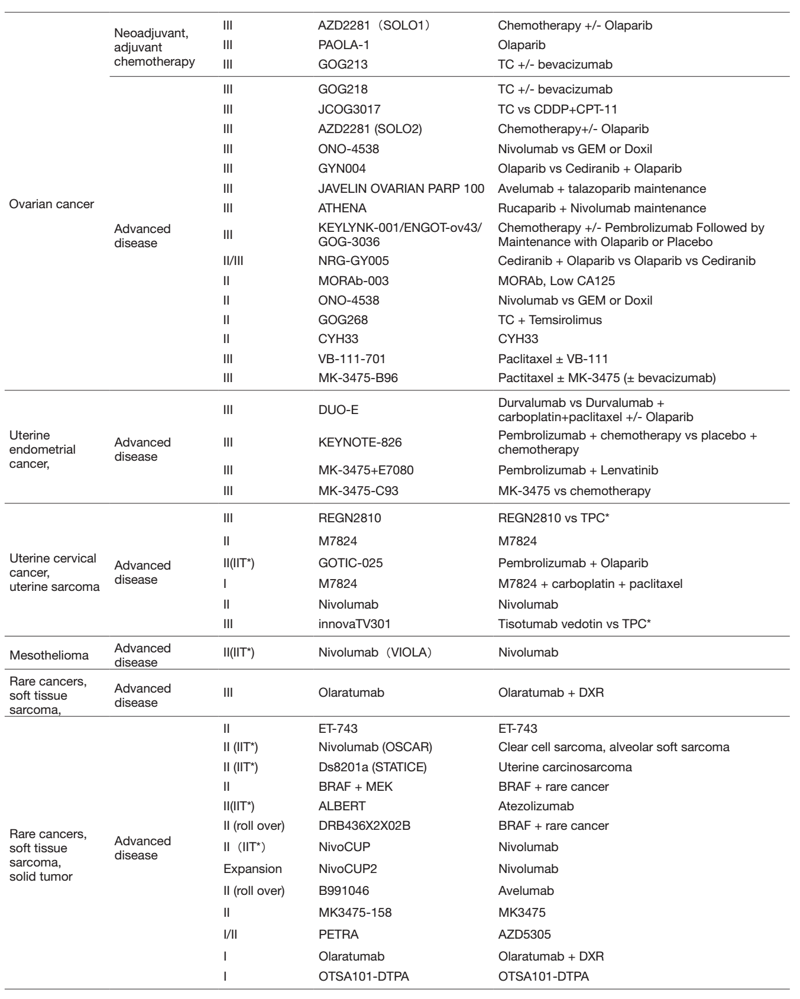
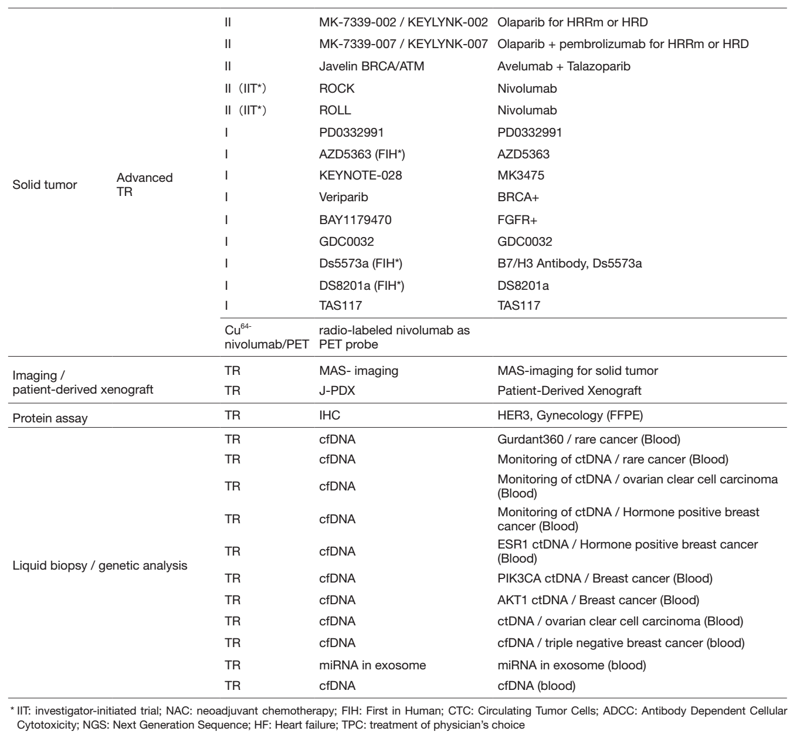
In 2021, we actively enrolled patients in phase I studies (including first in human or global) as well as domestic and international phase II and III studies (Table 2). Of note we enrolled patients in IITs for rare cancers. For example, we enrolled them in DS8201, monotherapy in carcinosarcoma patients with HER2 positive in rare tumor patients of all types, as an investigator-initiated clinical trial (IIT in Table 2). We also conducted many types of translational studies (TR) to find novel biomarkers.
Education
We provide rich educational opportunities to both residents and chief residents through clinical experience as well as research activities. Residents are encouraged to give presentations at local and national conferences. We vigorously support basic, clinical, and translational research conducted by postdoctoral researchers.
Future prospects
We will continue to establish new standard treatments and propose a near-future model of clinical management of adult solid tumors, including breast cancer, and gynecologic cancer. Moreover, we aim to build a comprehensive program, which includes tumor registry, translational research, clinical trials and patient care in rare adult tumors based on our rich clinical experience. We would also like to improve the efficiency of anti-cancer drug development by coordinating basic and translational research in early-phase clinical trials.
Table 2. Active Clinical Trials(Apr.2021- Mar, 2022)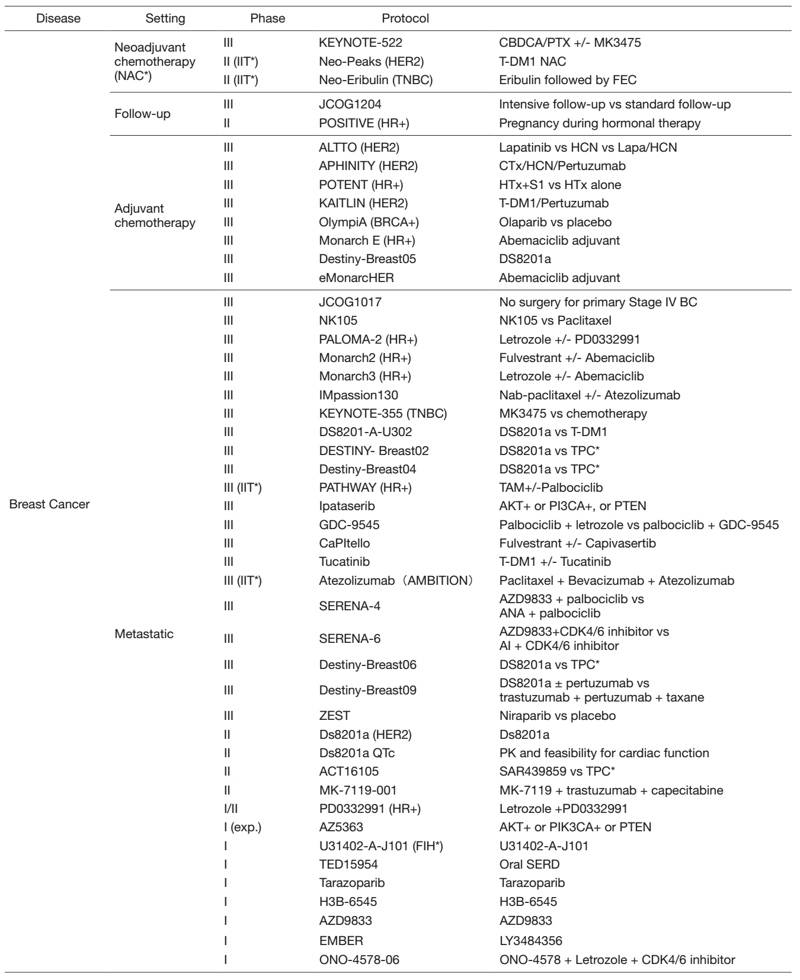

List of papers published in 2021
Journal
1. Ebata T, Yamashita S, Takeshima H, Yoshida H, Kawata Y, Kino N, Yasugi T, Terao Y, Yonemori K, Kato T, Ushijima T. DNA methylation marker to estimate ovarian cancer cell fraction. Med Oncol, 39:78, 2022
2. Okumura M, Du J, Kageyama SI, Yamashita R, Hakozaki Y, Motegi A, Hojo H, Nakamura M, Hirano Y, Okuma Y, Okuma HS, Tsuchihara K, Akimoto T. Comprehensive screening for drugs that modify radiation-induced immune responses. British journal of cancer, 126:1815-1823, 2022
3. Kaku S, Horinouchi H, Watanabe H, Yonemori K, Okusaka T, Boku N, Yamazaki N, Kawai A, Ohe Y, Kusumoto M. Incidence and prognostic factors in severe drug-induced interstitial lung disease caused by antineoplastic drug therapy in the real world. Journal of cancer research and clinical oncology, 2022
4. Shibayama T, Shimoi T, Mori T, Noguchi E, Honma Y, Hijioka S, Yoshida M, Ogawa C, Yonemori K, Yatabe Y, Yoshida A. Cytokeratin-positive Malignant Tumor in the Abdomen With EWSR1/FUS-CREB Fusion: A Clinicopathologic Study of 8 Cases. The American journal of surgical pathology, 46:134-146, 2022
5. Kirisawa T, Fukunaga A, Takamori H, Maejima A, Shinoda Y, Komiyama M, Fujimoto H, Yonemori K, Yoshida A, Matsui Y. Cytoreductive robot-assisted prostatectomy for systemic prostate rhabdomyosarcoma presenting as urinary retention. IJU case reports, 5:122-125, 2022
6. Yoshida A, Arai Y, Satomi K, Kubo T, Ryo E, Matsushita Y, Hama N, Sudo K, Komiyama M, Yatabe Y, Shibata T, Ichikawa H, Ichimura K, Kawai A, Mori T. Identification of novel SSX1 fusions in synovial sarcoma. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc, 35:228-239, 2022
7. Abe K, Maeda-Minami A, Ishizu T, Iwata S, Kobayashi E, Shimoi T, Kawano Y, Hashimoto H, Yamaguchi M, Furukawa T, Miyazaki S, Mano Y. Risk Factors for Hepatic Toxicity of High-dose Methotrexate in Patients With Osteosarcoma. Anticancer research, 42:1043-1050, 2022
8. Ishiki H, Hirayama T, Horiguchi S, Iida I, Kurimoto T, Asanabe M, Nakajima M, Sugisawa A, Mori A, Kojima Y, Udagawa R, Tsuchiya H, Oki M, Shimizu M, Yanai Y, Touma S, Nozawa K, Kojima R, Inamura N, Maehara A, Suzuki T, Satomi E. A Support System for Adolescent and Young Adult Patients with Cancer at a Comprehensive Cancer Center. JMA journal, 5:44-54, 2022
9. Makker V, Colombo N, Casado Herráez A, Santin AD, Colomba E, Miller DS, Fujiwara K, Pignata S, Baron-Hay S, Ray-Coquard I, Shapira-Frommer R, Ushijima K, Sakata J, Yonemori K, Kim YM, Guerra EM, Sanli UA, McCormack MM, Smith AD, Keefe S, Bird S, Dutta L, Orlowski RJ, Lorusso D . Lenvatinib plus Pembrolizumab for Advanced Endometrial Cancer. The New England journal of medicine, 386:437-448, 2022
10. Sato J, Shimizu T, Koyama T, Iwasa S, Shimomura A, Kondo S, Kitano S, Yonemori K, Fujiwara Y, Tamura K, Suzuki T, Takase T, Nagai R, Yamaguchi K, Semba T, Zhao ZM, Ren M, Yamamoto N. Dose Escalation Data from the Phase 1 Study of the Liposomal Formulation of Eribulin (E7389-LF) in Japanese Patients with Advanced Solid Tumors. Clinical cancer research: an official journal of the American Association for Cancer Research, 28:1783-1791, 2022
11. Takamizawa S, Shimoi T, Satomi-Tsushita N, Yazaki S, Okuya T, Kojima Y, Sumiyoshi-Okuma H, Nishikawa T, Tanioka M, Sudo K, Noguchi E, Yonemori K. Neutrophil-to-lymphocyte ratio as a prognostic factor for patients with metastatic or recurrent breast cancer treated using capecitabine: a retrospective study. BMC cancer, 22:64, 2022
12. Morita M, Shimomura A, Tokuda E, Horimoto Y, Kawamura Y, Ishizuka Y, Sekine K, Obayashi S, Kojima Y, Uemura Y, Higuchi T. Is adjuvant chemotherapy necessary in older patients with breast cancer? Breast cancer (Tokyo, Japan), 29:498-506, 2022
13. Kubo T, Arai Y, Sone M, Yonemori K, Abe O. Image-guided percutaneous needle biopsy for the diagnosis of cancer of unknown primary. Asia-Pacific journal of clinical oncology, 2022
14. Shimizu T, Kuboki Y, Lin CC, Yonemori K, Yanai T, Faller DV, Dobler L, Gupta N, Sedarati F, Kim KP. A Phase 1 Study of Sapanisertib (TAK-228) in East Asian Patients with Advanced Nonhematological Malignancies. Targeted oncology, 17:15-24, 2022
15. Mizuno T, Yoshida T, Sunami K, Koyama T, Okita N, Kubo T, Sudo K, Shimoi T, Ueno H, Saito E, Katanoda K, Shibata T, Yonemori K, Okusaka T, Boku N, Ohe Y, Hiroshima Y, Ueno M, Kuboki Y, Doi T, Nakamura K, Kohno T, Yatabe Y, Yamamoto N. Study protocol for NCCH1908 (UPFRONT-trial): a prospective clinical trial to evaluate the feasibility and utility of comprehensive genomic profiling prior to the initial systemic treatment in advanced solid tumour patients. Japanese journal of clinical oncology, 51:1757-1760, 2021
16. Yazaki S, Yoshida T, Kojima Y, Yagishita S, Nakahama H, Okinaka K, Matsushita H, Shiotsuka M, Kobayashi O, Iwata S, Narita Y, Ohba A, Takahashi M, Iwasa S, Kobayashi K, Ohe Y, Yoshida T, Hamada A, Doi T, Yamamoto N. Difference in SARS-CoV-2 Antibody Status Between Patients With Cancer and Health Care Workers During the COVID-19 Pandemic in Japan. JAMA oncology, 7:1141-1148, 2021
17. Hasegawa N, Kohsaka S, Kurokawa K, Shinno Y, Takeda Nakamura I, Ueno T, Kojima S, Kawazu M, Suehara Y, Ishijima M, Goto Y, Kojima Y, Yonemori K, Hayashi T, Saito T, Shukuya T, Takahashi F, Takahashi K, Mano H. Highly sensitive fusion detection using plasma cell-free RNA in non-small-cell lung cancers. Cancer science, 112:4393-4403, 2021
18. Noda-Narita S, Kawachi A, Okuyama A, Sadachi R, Hirakawa A, Goto Y, Fujiwara Y, Higashi T, Yonemori K. First-line treatment for lung cancer among Japanese older patients: A real-world analysis of hospital-based cancer registry data. PloS one, 16:e0257489, 2021
19. Okuma HS, Yonemori K, Kojima Y, Tanioka M, Sudo K, Noguchi E, Hijioka S, Wakakuwa K, Kato K, Hirakawa A, Kuchiba A, Kubo T, Ichikawa H, Yoshida A, Yatabe Y, Nakamura K, Mano H, Yamamoto N, Fujiwara Y. Clinical Utility of Circulating Tumor DNA in Advanced Rare Cancers. Frontiers in oncology, 11:732525, 2021
20. Sugawara S, Sone M, Itou C, Kimura S, Kusumoto M, Kato T, Yonemori K, Yatabe Y, Arai Y. Analysis of factors affecting the diagnostic yield of image-guided percutaneous core needle biopsy for peritoneal/omental lesions. Abdominal radiology (New York), 46:4499-4508, 2021
21. Shimoi T, Hashimoto J, Sudo K, Shimomura A, Noguchi E, Shimizu C, Yunokawa M, Yonemori K, Yoshida H, Yoshida M, Kato T, Kinoshita T, Fukuda T, Fujiwara Y, Tamura K. Hotspot mutation profiles of AKT1 in Asian women with breast and endometrial cancers. BMC cancer, 21:1131, 2021
22. Kawai A, Naka N, Shimomura A, Takahashi S, Kitano S, Imura Y, Yonemori K, Nakatani F, Iwata S, Kobayashi E, Outani H, Tamiya H, Naito Y, Yamamoto N, Doi T. Efficacy and safety of TAS-115, a novel oral multi-kinase inhibitor, in osteosarcoma: an expansion cohort of a phase I study. Investigational new drugs, 39:1559-1567, 2021
23. Monk BJ, Colombo N, Oza AM, Fujiwara K, Birrer MJ, Randall L, Poddubskaya EV, Scambia G, Shparyk YV, Lim MC, Bhoola SM, Sohn J, Yonemori K, Stewart RA, Zhang X, Perkins Smith J, Linn C, Ledermann JA. Chemotherapy with or without avelumab followed by avelumab maintenance versus chemotherapy alone in patients with previously untreated epithelial ovarian cancer (JAVELIN Ovarian 100): an open-label, randomised, phase 3 trial. The Lancet. Oncology, 22:1275-1289, 2021
24. Pujade-Lauraine E, Fujiwara K, Ledermann JA, Oza AM, Kristeleit R, Ray-Coquard IL, Richardson GE, Sessa C, Yonemori K, Banerjee S, Leary A, Tinker AV, Jung KH, Madry R, Park SY, Anderson CK, Zohren F, Stewart RA, Wei C, Dychter SS, Monk BJ. Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): an open-label, three-arm, randomised, phase 3 study. The Lancet. Oncology, 22:1034-1046, 2021
25. Saito A, Yoshida H, Nishikawa T, Yonemori K. Human epidermal growth factor receptor 2 targeted therapy in endometrial cancer: Clinical and pathological perspectives. World journal of clinical oncology, 12:868-881, 2021
26. Shimizu T, Fujiwara Y, Yonemori K, Koyama T, Sato J, Tamura K, Shimomura A, Ikezawa H, Nomoto M, Furuuchi K, Nakajima R, Miura T, Yamamoto N. First-in-Human Phase 1 Study of MORAb-202, an Antibody-Drug Conjugate Comprising Farletuzumab Linked to Eribulin Mesylate, in Patients with Folate Receptor-α-Positive Advanced Solid Tumors. Clinical cancer research: an official journal of the American Association for Cancer Research, 27:3905-3915, 2021
27. Suzuki M, Yagishita S, Sugihara K, Ogitani Y, Nishikawa T, Ohuchi M, Teishikata T, Jikoh T, Yatabe Y, Yonemori K, Tamura K, Hasegawa K, Hamada A. Visualization of Intratumor Pharmacokinetics of [fam-] Trastuzumab Deruxtecan (DS-8201a) in HER2 Heterogeneous Model Using Phosphor-integrated Dots Imaging Analysis. Clinical cancer research: an official journal of the American Association for Cancer Research, 27:3970-3979, 2021
28. Uchihara M, Tanioka M, Kojima Y, Nishikawa T, Sudo K, Shimoi T, Noguchi E, Maeshima AM, Yonemori K. Clinical management and outcomes associated with etoposide, doxorubicin, and cisplatin plus mitotane treatment in metastatic adrenocortical carcinoma: a single institute experience. International journal of clinical oncology, 26:2275-2281, 2021
29. Shimoi T, Nagai SE, Yoshinami T, Takahashi M, Arioka H, Ishihara M, Kikawa Y, Koizumi K, Kondo N, Sagara Y, Takada M, Takano T, Tsurutani J, Naito Y, Nakamura R, Hattori M, Hara F, Hayashi N, Mizuno T, Miyashita M, Yamashita N, Yamanaka T, Saji S, Iwata H, Toyama T. Correction to: The Japanese Breast Cancer Society Clinical Practice Guidelines for systemic treatment of breast cancer, 2018 edition. Breast cancer (Tokyo, Japan), 28:985-986, 2021
30. Yoshida H, Nishikawa T, Matsumoto K, Mori M, Hirashima Y, Takehara K, Ariyoshi K, Hasegawa K, Yonemori K. Histopathological features of HER2 overexpression in uterine carcinosarcoma: proposal for requirements in HER2 testing for targeted therapy. Virchows Archiv: an international journal of pathology, 478:1161-1171, 2021
31. Okagawa Y, Yoshinaga S, Noguchi E, Sekine S. Gastric metastasis from primary leiomyosarcoma of the broad ligament. Japanese journal of clinical oncology, 51:846-847, 2021
32. Hamanishi J, Takeshima N, Katsumata N, Ushijima K, Kimura T, Takeuchi S, Matsumoto K, Ito K, Mandai M, Nakai H, Sakuragi N, Watari H, Takahashi N, Kato H, Hasegawa K, Yonemori K, Mizuno M, Takehara K, Niikura H, Sawasaki T, Nakao S, Saito T, Enomoto T, Nagase S, Suzuki N, Matsumoto T, Kondo E, Sonoda K, Aihara S, Aoki Y, Okamoto A, Takano H, Kobayashi H, Kato H, Terai Y, Takazawa A, Takahashi Y, Namba Y, Aoki D, Fujiwara K, Sugiyama T, Konishi I. Nivolumab Versus Gemcitabine or Pegylated Liposomal Doxorubicin for Patients With Platinum-Resistant Ovarian Cancer: Open-Label, Randomized Trial in Japan (NINJA). Journal of clinical oncology: official journal of the American Society of Clinical Oncology, 39:3671-3681, 2021
33. Fujiwara K, Fujiwara H, Yoshida H, Satoh T, Yonemori K, Nagao S, Matsumoto T, Kobayashi H, Bourgeois H, Harter P, Mosconi AM, Vazquez IP, Reinthaller A, Fujita T, Rowe P, Pujade-Lauraine E, Ray-Coquard I. Olaparib plus bevacizumab as maintenance therapy in patients with newly diagnosed, advanced ovarian cancer: Japan subset from the PAOLA-1/ENGOT-ov25 trial. Journal of gynecologic oncology, 32:e82, 2021
34. Sugiyama Y, Hakuno D, Yonemori K, Endo J, Sueyoshi K. Paraneoplastic rheumatic syndrome caused by left ventricular intimal sarcoma. European heart journal. Cardiovascular Imaging, 22:e9, 2021
35. Shibayama T, Makise N, Motoi T, Mori T, Hiraoka N, Yonemori K, Watanabe SI, Esaki M, Morizane C, Okuma T, Kawai A, Ushiku T, Yatabe Y, Yoshida A. Clinicopathologic Characterization of Epithelioid Hemangioendothelioma in a Series of 62 Cases: A Proposal of Risk Stratification and Identification of a Synaptophysin-positive Aggressive Subset. The American journal of surgical pathology, 45:616-626, 2021
36. Nakamura IT, Ikegami M, Hasegawa N, Hayashi T, Ueno T, Kawazu M, Yagishita S, Goto Y, Shinno Y, Kojima Y, Takamochi K, Takahashi F, Takahashi K, Mano H, Kohsaka S. Development of an optimal protocol for molecular profiling of tumor cells in pleural effusions at single-cell level. Cancer science, 112:2006-2019, 2021
37. Abe S, Matsuzaki J, Sudo K, Oda I, Katai H, Kato K, Takizawa S, Sakamoto H, Takeshita F, Niida S, Saito Y, Ochiya T. A novel combination of serum microRNAs for the detection of early gastric cancer. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association, 24:835-843, 2021
38. Takeyasu Y, Okuma HS, Kojima Y, Nishikawa T, Tanioka M, Sudo K, Shimoi T, Noguchi E, Arakawa A, Mori T, Sunami K, Kubo T, Kohno T, Akihiko Y, Yamamoto N, Yonemori K. Impact of ALK Inhibitors in Patients With ALK-Rearranged Nonlung Solid Tumors. JCO precision oncology, 5:2021
39. Watase C, Shiino S, Shimoi T, Noguchi E, Kaneda T, Yamamoto Y, Yonemori K, Takayama S, Suto A. Breast Cancer Brain Metastasis-Overview of Disease State, Treatment Options and Future Perspectives. Cancers, 13:2021
40. Yamamoto N, Shimizu T, Yonemori K, Kitano S, Kondo S, Iwasa S, Koyama T, Sudo K, Sato J, Tamura K, Tomomatsu J, Ono M, Fukuda N, Takahashi S. A first-in-human, phase 1 study of the NEDD8 activating enzyme E1 inhibitor TAS4464 in patients with advanced solid tumors. Investigational new drugs, 39:1036-1046, 2021
41. Kondo S, Shimizu T, Koyama T, Sato J, Iwasa S, Yonemori K, Fujiwara Y, Shimomura A, Kitano S, Tamura K, Yamamoto N. First-in-human study of the cancer peptide vaccine TAS0313 in patients with advanced solid tumors. Cancer science, 112:1514-1523, 2021
42. Yonemori K, Shimizu T, Kondo S, Iwasa S, Koyama T, Kitano S, Sato J, Shimomura A, Shibaki R, Suri A, Kase Y, Sumino S, Tamura K, Yamamoto N. The safety, tolerability and pharmacokinetics of niraparib in Japanese patients with solid tumours: results of a phase I dose-escalation study. Japanese journal of clinical oncology, 51:693-699, 2021
43. Kitadai R, Shimoi T, Sudo K, Noguchi E, Nagata Y, Sawada R, Takashima A, Boku N, Yonemori K. Efficacy of second-line treatment and prognostic factors in patients with advanced malignant peritoneal mesothelioma: a retrospective study. BMC cancer, 21:294, 2021s
44. Fujiwara Y, Kenmotsu H, Yamamoto N, Shimizu T, Yonemori K, Ocampo C, Parikh A, Okubo S, Fukasawa K, Murakami H. Phase 1 study of telisotuzumab vedotin in Japanese patients with advanced solid tumors. Cancer medicine, 10:2350-2358, 2021
