Annual Report 2021
Department of Laboratory Medicine
Hiromichi Matsushita, Kuniko Sunami, Minoru Kojima, Takahiro Nishino, Naoki Maezawa, Motoi Miyakoshi, Chiaki Hayashi, Yoji Hashimoto, Yasuo Shibuki, Hiroki Kakishima, Koji Yamada, Rie Matsuo, Kazuya Tokita, Chiaki Ikeda, Shuji Ota, Arisa Hanai, Yusuke Okui, Sayaka Takeuchi, Jun-ichi Kubo, Kyosuke Tosawa, Sachiko Kobayashi, Kayo Tei, Satoe Miyaki, Noriko Takahashi, Tomoe Ito, Kyoko Orihara, Akino Kino, Takako Takada, Kyoko Osanai, Ruriko Machida, Mayu Shimono, Fumie Yoda, Hiroshi Chigira, Yu Aruga, Saori Kobayashi, Kaori Yamaguchi, Saori Nakabayashi, Shingo Nakajima, Hideya Matsubayashi, Saeko Shirahama, Akiko Takayanagi, Mei Fukuhara, Kumi Nakatani, Moemi Kasane, Kazuhiro Yoshida, Kenta Takehara, Madoka Kondo, Kana Katsuragi, Aisa Mizoguchi, Nao Iwashita, Sakura Ishida, Misato Tsubokura, Haruka Katagiri, Ayaka Ichikawa, Yuka Yasuno, Yuki Minakawa, Kanako Kanaizuka, Haruka Hirakochi, Minami Sato, Kaho Matsui, Takashi Kubo, Mayuko Kitami, Naoko Fujisawa, Shigeru Tamura, Megumi Masuda, Hiyori Yatsu, Yuri Tanaka, Kana Miyajima, Misaki Sato, Chika Tokutake, Dai Mikami, Aya Mikami, Haruki Mitsuya, Sakiho Takeoka, Hinako Nakamura, Nozomi Shishido, Manami Ito, Juri Hiraiwa, Nao Saito, Yusuke Takai, Aya Iwasaki, Yuma Sekiguchi, Ginga Nagasawa, Yoshiko Shibata, Ritsuko Toyama, Chieko Nozawa, Kazue Naoi, Kinue Tsubokawa, Kozaburo Endo
Introduction
The work performed at the Department of Laboratory Medicine includes clinical laboratory testing (urinalysis and other general testing, biochemistry, immunology, hematology, bacteriology, and gene testing), blood sampling, transfusion and cell therapy testing, physiological function examinations (ultrasonography, electrocardiograms, and respiratory function tests), and pathological examinations. The Department has maintained accreditation status under ISO 15189, the international standard defining the requirements for quality and competence in medical laboratories, since September 2012. In 2021, we underwent a periodic inspection that found no severe problems.
The number of clinical laboratory test orders increased to 7,094,537 in 2021, corresponding to 110.7 % of the figure in 2020. This is the same level as the pre-COVID-19 era (Table 1).
Table 1. Number of clinical tests performed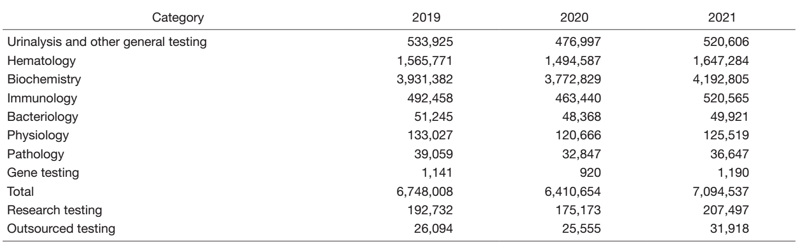

Routine activities
The Clinical Laboratory Testing Section is carrying out job rotation of staff in biochemistry and immunology to improve work performance and efficiency, which contributes to managing the increasing number of laboratory tests in clinical trials and practice. In hematology, lots of flow cytometry tests have been performed to detect minimal residual disease in various hematopoietic malignancies as well as analyses of small amounts of specimens such as cerebrospinal fluid. In bacteriology, the genetic test to detect the COVID-19 virus has been applied not only for all the scheduled surgical operations but also for emergency ones. In gene testing, we have been handling many cases in the expert panel meetings for cancer genomic medicine as a core hospital, based on the results of cancer gene panel sequencing; the number of cases examined has been increasing, partially due to the application of our health insurance for liquid specimens (Table 2). In blood sampling, we have been working to maintain the shortened patient waiting time while prioritizing medical safety, aiming at less than 20 minutes for 85% of patients.
Table 2. Number of sessions and cancer genomic profiling tests
in expert panel meetings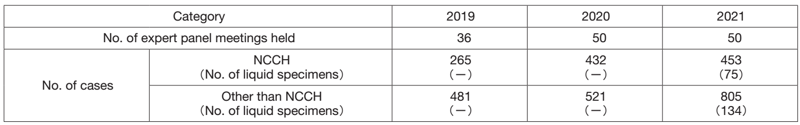

The Blood Transfusion Testing and Cell Therapy Section has automated the evaluation of blood types in shift duties and the processing of ascites in cell-free and concentrated ascites reinfusion therapy (CART), and managed the cell processing in chimeric antigen receptor T-cell (CAR-T) therapy, which has been gradually increasing.
The Physiological Examination Section has actively performed evaluations of cardiotoxicity of anticancer agents and sinusoidal obstruction syndrome / veno-occlusive disease (SOS/VOD) after hematopoietic stem cell transplantation.
The Pathological Examination Section has introduced an automated system for liquid-based cytology (LBC) in gynecological diseases for more efficient processing.
Research activities
The Department of Laboratory Medicine is conducting basic and clinical studies concerning factors affecting the accuracy of laboratory tests, and has reported novel findings related to the daily work. In biochemistry and immunology, we have been studying the stability and clinical use of specific tumor marker tests in collaboration with related departments and manufacturers. In hematology, we performed an evaluation of an automated analyzer for a blood coagulation system in collaboration with a manufacturer. Our activities related to cancer genome profiling tests contribute to clinical studies in National Cancer Center Hospital, as well as decisions in cancer genomic medicine in Japan. The Blood Transfusion Testing and Cell Therapy Section has reported on its experience of processing in CART, and contributed to the clinical studies of CAR-T therapy.
Education
Medical technologists are required to provide precise results of laboratory tests and examinations in each area of laboratory medicine. Human resource development is therefore conducted according to the education and training protocol prescribed in ISO 15189. Furthermore, the Department of Laboratory Medicine aims to cultivate the skills and knowledge of medical technologists by actively helping them pass examinations and supporting their other academic activities. In particular, in 2021, a laboratory technician received the qualification “Specialist in Hematology” from the College of Laboratory Medicine of Japan. We have received visits from many students aiming to become medical technologists and have inspired in them an interest in laboratory medicine. In addition, a resident who aims to become a specialist in laboratory medicine is currently in training.
Future Prospects
As an ISO 15189-accredited testing facility, we guarantee that our quality and capabilities meet international standards and also promote cooperation in clinical trials and clinical studies. In the near future, we will modify the system of the Clinical Laboratory Testing Section to conduct laboratory tests more efficiently using automated analyzers. Additionally, we will increase the personnel who specialize in cell therapy and flow cytometric analyses through professional education, in order to expand our capacity for these operations. Regarding cancer genomic medicine, we will continue to manage the expert panel meetings as in previous years, in spite of the increased number of cancer genome profiling tests. Through carrying out these tasks, we will establish a clinical laboratory that can contribute to the valuable information provided by each clinical department as well as providing it ourselves.
List of papers published in 2021
Journal
1. Kojima N, et al. Co-expression of ERG and CD31 in a subset of CIC-rearranged sarcoma: A potential diagnostic pitfall. Modern pathol, in press
2. Tsubokura M, et al. Adverse effects of cell free and concentrated ascites reinfusion therapy for malignant ascites: A single-institute experience. BMC Cancer 2022; 22: 268.
3. Hori Y, et al. Cerebrospinal fluid infiltration of primary cutaneous gamma delta T-cell lymphoma. eJHaem 2022; 3(1): 239-40.
4. Aruga Y, et al. Convenience of Hgb-O detected by optical method in XN-series hematology analyzers in evaluating hemoglobin concentration in samples with chylous turbidity. Sci Rep 2021; 11(1): 14978.
5. Hara R, et al. Association between measurable residual disease kinetics and outcomes of Philadelphia chromosome-positive acute lymphoblastic leukemia. Ann Hematol 2021; 100(10): 2479-2486.
6. Tanaka Y, et al. Multi-omic profiling of peritoneal metastases in gastric cancer identifies molecular subtypes and therapeutic vulnerabilities. Nat Cancer 2021; 2: 962-977.
7. Yazaki S, et al. Difference in SARS-CoV-2 antibody status between patients with cancer and health care workers during the pandemic in Japan. JAMA Oncol 2021;7(8):1141-1148.
8. Hasegawa M, et al. Evaluation of the performance, operability, and safety of Plasauto µTM, a new type of machine for cell-free and concentrated ascites reinfusion therapy (CART), in a post-marketing clinical study. Ther Apher Dial 2021; 25(4): 407-414.
9. Kasane M, et al. Usefulness of hematopoietic progenitor cell monitoring to predict autologous peripheral blood stem cell harvest timing: A single-center retrospective study. Transfus Apher Sci 2021; 60(4): 103150.
10. Kojima M, et al. Black ascites. QJM 2021; 114(7): 523-524.
11. Sunami K, et al. Chronological improvement in precision oncology implementation in Japan. Cancer Sci. 2022 doi: 10.1111/cas.15517
12. Kubo T, et al. Co-expression of ERG and CD31 in a subset of CIC-rearranged sarcoma: a potential diagnostic pitfall. Mod Pathol. 2022 Apr 19. doi: 10.1038/s41379-022-01078-8.
13. Sunami K, et al. Cancer Sci. 2022 Jul 25. doi: 10.1111/cas.15504
14. Sunami K, et al. Clinical Application of Comprehensive Genomic Profiling Tests for Diffuse Gliomas Cancers (Basel) 2022 May 16;14(10):2454.
15. Kubo T, et al. Central nervous system sarcoma with ATXN1::DUX4 fusion expands the concept of CIC-rearranged sarcoma. Genes Chromosomes Cancer. 2022 Jun 17. doi: 10.1002/gcc.23080.
