Annual Report 2021
Department of Cellular Therapy Processing
Takahiro Fukuda, Minoru Kojima, Takashi Tanaka
Introduction
The Department of Cellular therapy processing was established in 2020 as a shared department at NCCH to handle appropriately the increasing need for cellular therapy in recent years. We support cell collection, preparation, and storage not only for hematopoietic cell transplantation (HCT) but also for new cellular therapies such as Chimeric Antigen Receptor T-cell (CAR-T) therapy.
The Team and What We Do
Our department consists of 3 physicians, 11 clinical engineers, and 6 medical technicians. In fiscal 2021, the number of cell collections was 32 for autologous peripheral blood stem cell (PBSC), 27 for related PBSC, 9 for unrelated PBSC, and 8 for unrelated bone marrow from Japan Marrow Donor Program (JDMP) donors (Table 1). We performed 25 related peripheral blood stem cell transplantations (PBSCTs) (including 14 HLA-haploidentical), 1 related bone marrow transplantation (BMT), 24 unrelated PBSCTs, 3 unrelated BMTs, 18 cord blood transplantations (CBTs), and 31 autologous PBSCTs. The COVID-19 pandemic has caused a delay in coordination through the JMDP, but the use of HLA-haploidentical transplantation and CBT compensated for the delay and contributed to maintaining the number of transplantations.
As for CAR-T therapy, we started full-fledged operation of Kymriah® and the number of Kymriah® cases was increased. In addition, Breanji® was approved as a new CAR-T therapy in March 2021 in Japan, and we performed Breanji® for 7 patients.
As for TEMCELL®, which is an off-the-shelf bone marrow-derived mesenchymal stem cell treatment for refractory acute graft-versus-host disease (GVHD), we are responsible for cell storage and preparation. The number of preparations increased three-fold in fiscal 2021, so we made a substantial contribution to the treatment of severe acute GVHD.
Table 1. Number of each type of procedure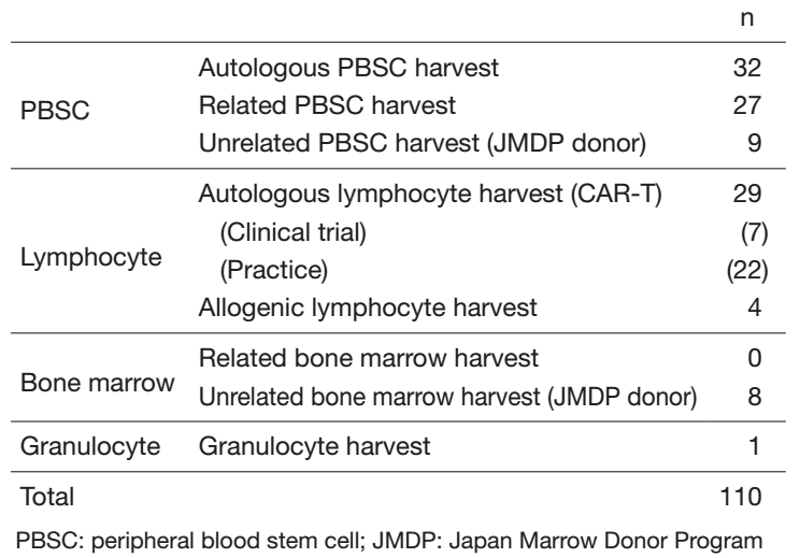

Research activities
We reviewed blood product use in 729 consecutive allogeneic HCT recipients at our center to assess the volume of red blood cells (RBCs) and platelets required after allogeneic HCT. We revealed that related PBSCT required a significantly lower RBC transfusion volume by day 30 compared to unrelated BMT. PBSCT from haploidentical related donors and CBT required a significantly greater RBC transfusion volume. For platelet transfusion, related and unrelated PBSCT required a significantly lower volume than unrelated BMT, and CBT a greater volume (Kurosawa IJH 2021). In the annual meeting of the Japan Society of Transfusion Medicine and Cell Therapy, we presented a clinical course of a patient who was naive for Hepatitis B virus and underwent allogeneic HCT from a donor who had been vaccinated for Hepatitis B (Iwashita JSTMCT 2021).
Clinical trials
We are involved in clinical trials of CAR-T therapies. The numbers of autologous lymphocyte harvests in clinical trials were 2 in the Department of Experimental Therapeutics (TAK-102, TAK-103) and 5 in the Department of Hematology (JCAR017).
Education
We have trained 1 clinical engineer to develop the skills for blood separator operation. We have also trained several certified apheresis nurses who belong to the Japanese Red Cross Society to develop their skills for PBSC harvest.
Future Prospects
A new CAR-T therapy, Yescarta®, was approved in Japan in 2021 and our hospital will be certified as a treatment facility in 2022. Along with HCT, CAR-T therapy is expected to increase in the future. It is expected that the role of our department will increase more and more, and we need to expand our system in order to provide appropriate treatment for all patients.
List of papers published in 2021
Journal
1. Tsubokura M, Adegawa Y, Kojima M, Tanosaki R, Ohtake R, Kase Y, Iwashita N, Kasane M, Nakabayashi S, Takeuchi S, Kato K, Boku N, Kanemitsu Y, Okusaka T, Fujimoto H, Yonemori K, Ishiki H, Kawamura K, Satomi E, Matsushita H. Adverse effects of cell-free and concentrated ascites reinfusion therapy for malignant ascites: a single-institute experience. BMC cancer, 22:268, 2022
2. Kasane M, Kurosawa S, Kojima M, Iwashita N, Kase Y, Tsubokura M, Nakabayashi S, Ikeda C, Kawamura K, Matsushita H, Narita R, Fukumoto H, Fujino T, Makita S, Fukuhara S, Munakata W, Suzuki T, Maruyama D, Ito A, Tanaka T, Inamoto Y, Kim SW, Tajima K, Tanosaki R, Izutsu K, Fukuda T. Usefulness of hematopoietic progenitor cell monitoring to predict autologous peripheral blood stem cell harvest timing: A single-center retrospective study. Transfusion and apheresis science: official journal of the World Apheresis Association: official journal of the European Society for Haemapheresis, 60:103150, 2021
3. Kurosawa S, Yamaguchi T, Nakabayashi S, Kasane M, Tsubokura M, Iwashita N, Minakawa Y, Ohtake R, Kawamura K, Nishioka Y, Takeda W, Hirakawa T, Aoki J, Ito A, Tanaka T, Inamoto Y, Kim SW, Kojima M, Takanashi M, Fukuda T. Effect of donor type on volume of blood transfusions required after allogeneic hematopoietic cell transplantation. International journal of hematology, 113:518-529, 2021
4. Kojima M, Namikawa K, Kase Y, Matsushita H. Black ascites. QJM: monthly journal of the Association of Physicians, 114:523-524, 2021
