Center for Research Administration and Support (CRAS)
Teruhiko Yoshida, Nobuko Ushirozawa, Satoshi Takeuchi, Sachie Senda, Kiyokazu Kawashima, Yuichi Amanuma, Fumie Suzuki, Kiyoka Nagayama, Yuko Yamada, Sawako Nakayama, Emi Nobuhara, Anne Mochizuki, Genta Ohno, Yukari Nakayama, Ryota Kosaka, Junko Kaneko, Kyoko Chiba, Maho Muraoka, Kazunori Aoki, Kazuhiko Aoyagi, Izumi Hayama, Noriko Yamashita, Yayoi Ofuji, Kuniko Takahashi, Norie Enomoto, Yoko Yokota, Satoko Arai, Emi Kunimatsu, Keiko Koide, Tomoko Ishida, Sanae Kawada, Masami Sekine, Masahiko Ozaki, Yuki Harada, Takahiro Sakai, Rika Oumi, Hayato Kamata, Kaori Yanagisawa, Chiemi Notake, Seiko Kondou, Sayaka Yamamoto, Yoko Matsubuchi, Taro Shibata, Aya Kuchiba, Junki Mizusawa, Masashi Wakabayashi, Gakuto Ogawa, Ryunosuke Machida, Ryo Sadachi, Chihiro YAITA, Yuki Konda, Naomi Konishi, Kohei Uemura, Ryo Kitabayashi, Kanako Fuyama, Akihiro Hirakawa, Shogo Nomura, Seiichiro Yamamoto, Suga Yamagami, Masashi Mikami, Shintaro Iwamoto, Ryo Kanzaka, Kenji Matsui, Tsunakuni Ikka, Haruka Nakada, Tomoko Seiki
Introduction
The Center for Research Administration and Support (CRAS) was established on July 16, 2014. The starting members of the CRAS were approximaztely 160 staff, who togethtter offered diverse functions and specialties, ranging from research fund administration, alliances with the private sector, intellectual properties, clinical research coordinators and data managers, monitoring and audit, biostatistics support, offices for research ethics (IRB), and COI committees.
The background and purpose of the creation of the CRAS was explained by Dr. Tomomitsu Hotta, the President of the National Cancer Center (NCC), in NCC News 2014 Vol. 5 No. 3 (in Japanese). Briefly, since its foundation in 1962, the NCC has added several new segments and organizations to evolve as a comprehensive cancer center. Because each segment needed its own research infrastructure, support activities in the NCC had become fragmented and scattered with the possibility of gaps and redundancies. Dr. Hotta approached the Strategic Planning Bureau and assembled the “NCC New Vision” in FY 2014, in which he proposed integration and communication for various research support functions in the NCC. The CRAS was created in response to the FY 2014 Vision.
In FY 2015, the NCC Hospital (NCCH) and the NCC Hospital East (NCCHE) were certified as Core Clinical Research Hospitals under the Medical Care Act in August and September, respectively. As a result, the governance of the Research Coordination Division, Research Promotion Division and Regulatory Science Section of the CRAS has moved to the Clinical Research Support Offices, which belong to the common departments of each hospital. In January 2017, these divisions and section were officially separated from the CRAS in the NCC organization.
Another major reformation of the CRAS in FY 2017 was the establishment of the Bioethics Division by expanding the former Bioethics Section. The Clinical Trials Act was promulgated on April 14, 2017 and came into effect on April 1, 2018. Despite the general scarcity of human resources in the specialty field, the NCC has been endowed with strong staff, who have been contributing to work not just inside the NCC but also all over Japan with regard to research ethics-related issues including the implementation of the Clinical Trial Act.
In FY 2018, the concept of the RA (research administrator) system in the NCC was discussed in the CRAS and updated in the NCC headquarters.
In FY 2019, under the leadership of the new Chief of Bioethics Division, several important discussions and pieces of rule-making were done in collaboration with the researchers and staff in both campuses of the NCC. For instance, liaison and research manager staff have been assigned in each section of the NCC to facilitate communication between researchers and the research ethics review committee and build a solid base for the research ethics in the research groups.
In FY 2020, the CRAS continued to work in response to directions by the government on research governance, such as the establishment of a basic policy for the data sharing in collaboration with the JH (Japan Health Research Promotion Bureau), an organization officially established in FY 2020 to promote alliance among the 6 National Centers. The CRAS led the coordination of various sections on both campuses to get a more precise and comprehensive picture of the research-related funding and revenue of the NCC.
In FY 2021, the NCC achieved the highest amounts for total public grants awarded to its researchers and revenues from intellectual properties. In response, the CRAS has made several renovations to support the researchers, such as the introduction of electronic grant application systems, so that they can find more time and energy to concentrate on their research. Another revision of the CRAS organization scheme was moving the Human Research Protection Section from the Bioethics Division to the Research Administration Division, thereby demarcating the research review function and research support services such as research ethics consultation.
(Future prospects)
The NCC embarked on the new era under the leadership of a newly appointed President and Directors of the NCC Research Institute (NCCRI) and both hospitals (NCCH, NCCHE) on April 1, 2016. The Vision and high-priority research targets have been redefined, and re-organization remains in progress in various parts of the NCC. However, the core concept of the CRAS stays unchanged to contribute to the NCC mission by promoting organic unity of the NCC as a whole. It is particularly important to respect differences in roles and characteristics of each NCC campus, Tsukiji and Kashiwa, to make the most of their strong points to maximize the research output of the NCC. Collaborations with the Clinical Research Support Offices of both hospitals remain crucial, and the CRAS aims to contribute to bringing the various sections together in the NCC.
1. Research Administration Division
1) Research Administration Section
The Research Administration Section is a central office in charge of various administrative work related to research funding including application and reporting. The major external funding sources of the NCC are competitive grants from the government and government-supported agencies, such as the Ministry of Health, Labour and Welfare (MHLW), the Japan Society for the Promotion of Science (JSPS), and the Japan Agency for Medical Research and Development (AMED).
This section also serves as an administrative office for the NCC Research and Development Fund, which is provided directly from the government to the NCC for fulfillment of its mission as the national core institute of cancer control. This section organized seminars regarding research funding and its rules to prevent financial misconduct or research misconduct.
The Guidelines for Managing and Auditing Public Research Funds at Research Institutes were updated by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) in February 2014 and adopted by the MHLW in March 2014. This section serves as a compliance promotion office of the Guidelines and has established a new system for research fund administration, which is fully compatible with the new Guidelines.
The Guidelines for Managing and Auditing Public Research Funds at Research Institutes was updated by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) in February 2014 and adopted by the MHLW in March 2014. This section serves as a compliance promotion office of the Guidelines and has established a new system for research fund administration, which is fully compliant with the new Guidelines.
Based on revision by MEXT of the guidelines for managing and auditing public research funds at research institutions, we formulated an action plan to prevent the improper use of research funds. As part of our enlightenment activities, we distributed newsletters and disseminated items related to the prevention of improper use of research funds. (The items were reported by the compliance promotion officers of each department to each department’s members, in order to raise their awareness related to the prevention of financial misconduct.)
From July 2021, each research application process has been available online. As a result, it has become possible to make research applications online instead of using paper documents, and this change has lead to significant improvement in efficiency of work for both researchers and office workers.
2) Research Alliance Section and Intellectual Property Section
We efficiently build bridges between NCC research and industries by collective management of the collaborative research alliances and utilization of intellectual properties (IPs) arising therefrom.
1. Alliance with the private sector
We have supported the NCC’s wide variety of alliances, including but not limited to establishing worldwide comprehensive collaborative partnerships with major pharmaceutical companies and academic institutions. Currently, 11 comprehensive collaborative alliances are active between the NCC and domestic/overseas industry partners (Fig. 1). We have also been supporting a nationwide genomic screening project (SCRUM-Japan “Cancer Genome Screening Project for Individualized Medicine in Japan”) led by the NCC as a challenge being undertaken first in Japan. Nationwide medical institutions and 21 domestic/overseas pharmaceutical companies have joined the aforementioned project (Table 1). The collaborative research and research funds that we manage have been increasing year by year (Fig. 2). In 2021, the number of collaboration cases reached 582, and the amount of research funds reached 3.75 billion Japanese yen.
2. Intellectual property management
We encourage NCC researchers not only to obtain collaborative research results with companies (needless to say), but also to license-out IPs derived from their research at an early stage to partner companies who can work together with them towards realization of their research results. Our IP strategy enables us to reduce costs in patent prosecution and to expedite commercialization of our research results, eventually enabling the receipt of monetary remuneration. On the other hand, for IPs the NCC substantially bears the cost of, we constantly review their value and dismiss those that cannot find sponsors within a certain period. By allocating its financial resources to IPs with commercial value, the NCC has been successful in sustaining positive outcomes from IP management for years (Fig. 3).
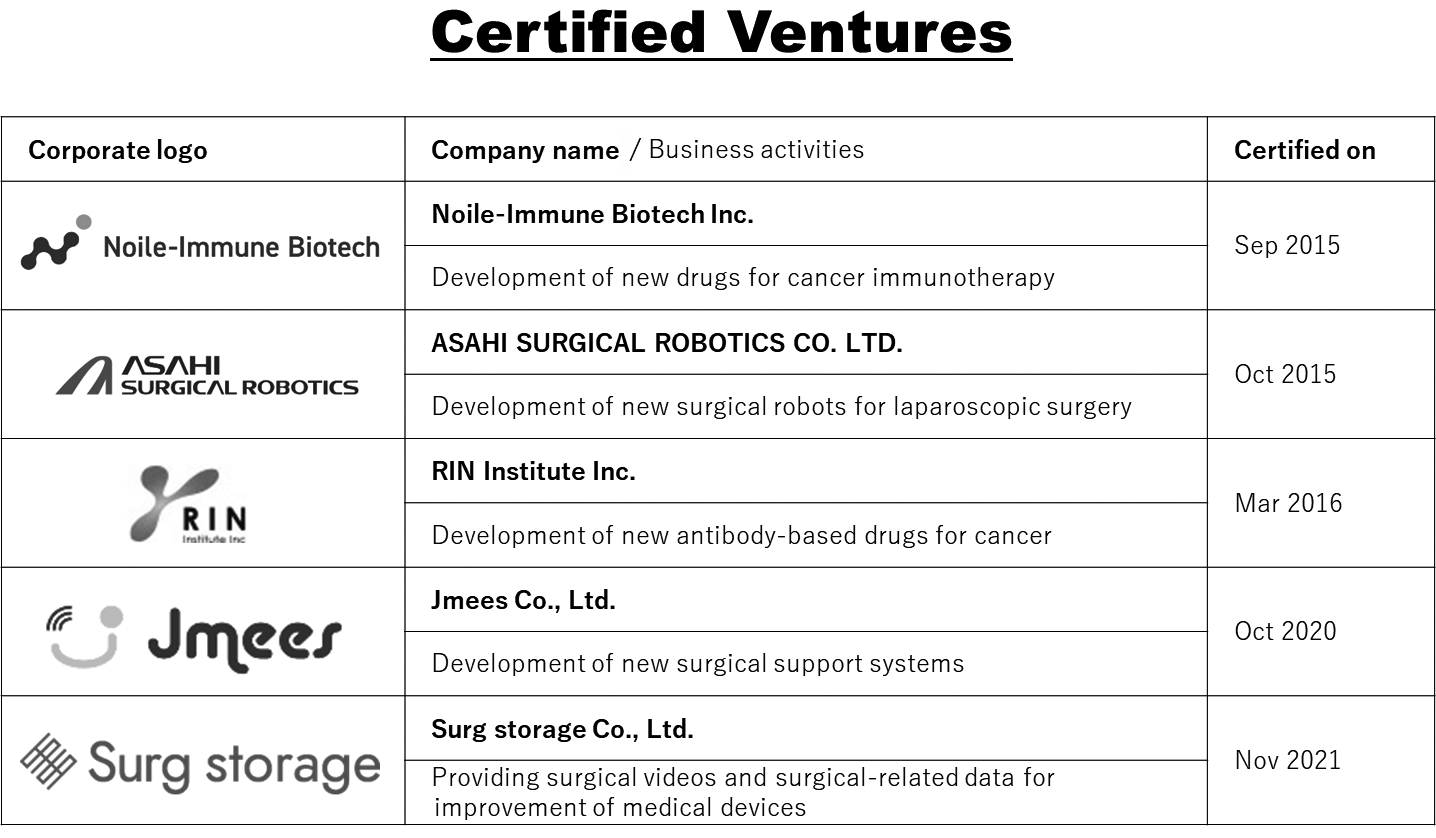
3. Certified ventures
We certify “NCC-launched venture companies” that seek to apply and commercialize IPs and research results originating from the NCC. As of 2021, we have certified five NCC-launched venture companies (Table 2).
Figure 1. Comprehensive Research Alliance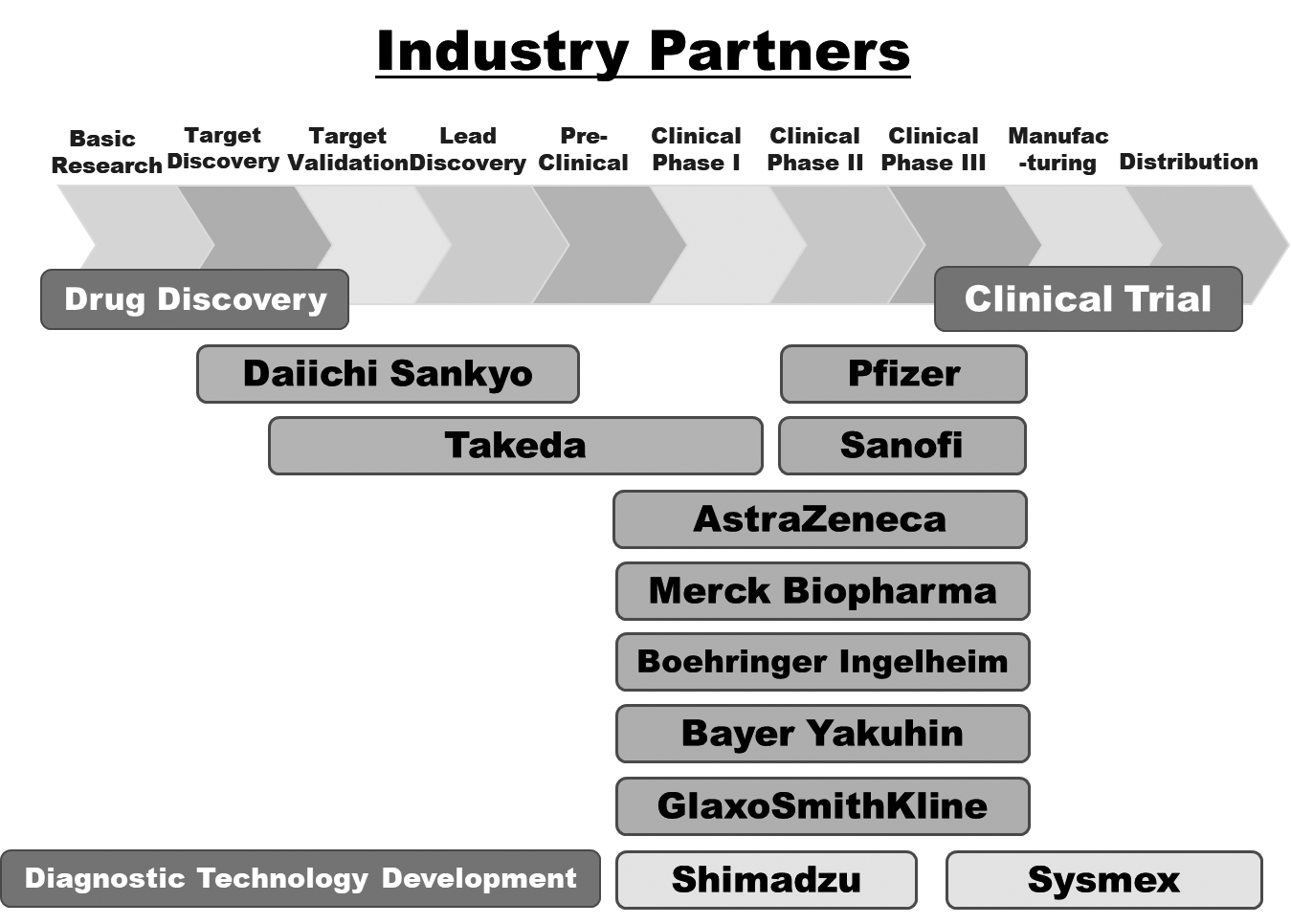

Table 1. Participant Companies in SCRUM-Japan

Figure 2. Collaborative Research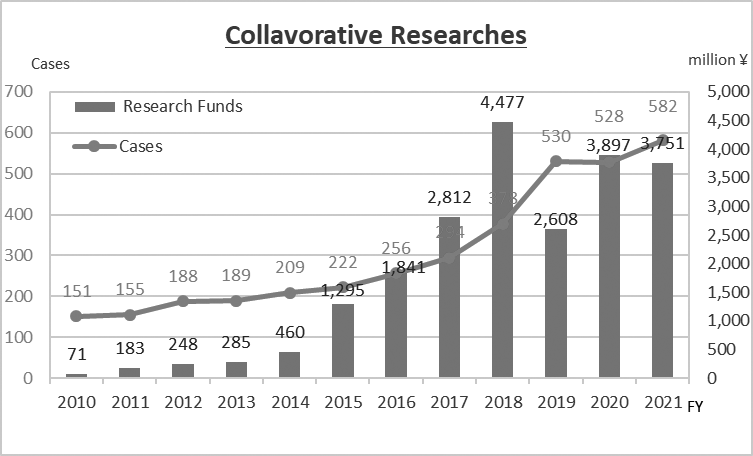

Figure 3. Intellectual Property Income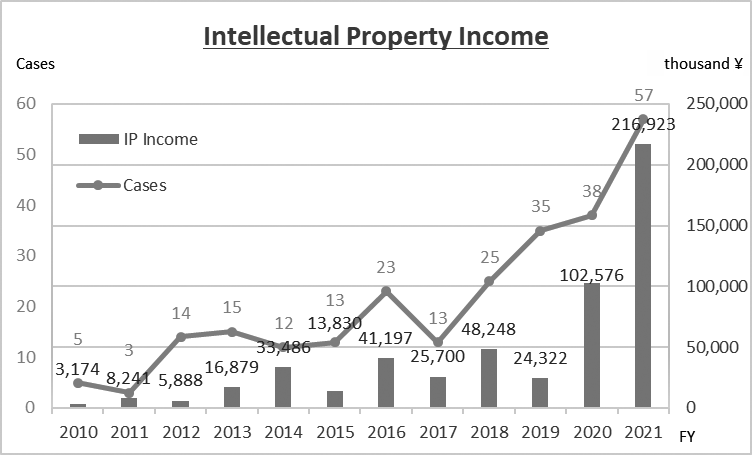

Human resource development and education
We host educational seminars on essential IP knowledge for NCC faculty members at least twice a year. Our staff are encouraged to participate in seminars and conferences to update their knowledge on IP laws, regulations, and guidezlines as well as scientific technologies, all of which are necessary to promote academia-industry alliances. From time to time, we consult with experts, such as lawyers and patent attorneys, to solve problems and to gain practical experience.
Prospects
Under the NCC’s vision of “commitment to providing the best possible cancer treatment and care through relentless partnership with the community”, we will keep supporting the creation of effective and innovative collaborative research frameworks which develop into fruitful research partnerships. We will continue to play key roles in assisting the NCC with making decisions on its IP management.
3) Research Administrators
Research administrators (RAs) have supported translating research outcomes into practical applications based upon a comprehensive alliance with pharmaceutical companies. As part of the Tsukiji TR Board’s activity, RAs promoted co-clinical studies in cooperation with industries by holding a briefing meeting and exhibition in BioJapan 2021 targeted at companies to introduce the NCC’s bioresources and novel technologies. RAs also supported the progress of AMED research projects through the Practical Research for Innovative Cancer Control Management Office (PRIMO), and the 6 National Centers (NCs) cooperated on research projects related to the Japan Health Research Promotion Bureau (JH).
(Future Prospects)
RAs promote the translation of innovative research in the NCC to clinical diagnostic and therapeutic development and to patient care through several major mechanisms: the funding activities in the NCC (Seeds Selection Committee and Center for Promotion of Translational Research (CPOT)), comprehensive alliances with leading companies, participation in the academic Drug Discovery Network, and cooperation between the 6 NCs and JH.
4) Human Research Protection Section
The Human Research Protection Section (HRPS) serves as the administrative offices for the various types of IRBs for human subject research, including the certified review committees defined by the Clinical Trials Act (Act No. 16 of April 14, 2017).
The accomplishments of the reviews by the IRBs at the NCC in FY 2020 included 2,069 active research projects and 434 new research plans by the NCC faculty members (including 36 central reviews), while a total of 23 new research plans by researchers outside of the NCC were requested for review.
(Future Prospects)
The HRPS will continue working, in cooperation with the NCCH’s and the NCCHE’s Ethical Review Support Sections, to ameliorate the NCC’s whole ethics review processes for more appropriate and efficient ways to achieve goals. Particularly in FY 2022, the HRPS will adjust and manage the institutional ethics review system for the new ethics guidelines which are scheduled to be enforced on April 1st, 2022.
2. Biostatistics Division
The Biostatistics Division has a role of responsibility in formulating research hypotheses, study design, analysis, interpretation and publication, especially in JCOG and EPOC clinical trials and the investigator-initiated clinical trials which are led by investigators in the NCC Hospitals. We have also committed ourselves to establish an infrastructure to support the clinical trials in the NCC Hospitals. Furthermore, we have been actively involved in collaborative relationships with the NCC Research Institute and the Institute of Cancer Control.
Furthermore, we have provided biostatistical consultation and expertise, which supports NCC investigators working on basic, translational, clinical and epidemiological research. We offered advice for about 149 problems (75 in Tsukiji campus and 74 in Kashiwa campus) for which biostatistical consultation was requested from April 2020 to March 2021.
Education
We provided 13 introductory biostatistics lectures for investigators in the NCC to learn and review the elementary aspects of biostatistics. We had a cumulative total of 728 participants. In addition, we hosted a biostatistics lecture to cover advanced and important biostatistical sides of various application fields and a total of 86 investigators participated. The lectures are open to any applicants from outside institutes.
In addition, we provided an internship program for 16 graduate students from the graduate schools of the University of Tokyo (the biostatistics and bioinformatics course, Graduate School of Interdisciplinary Information Studies).
(Future Prospects)
The NCC has a critical role for providing clinical services and education, conducting research and making policy recommendations/proposals, which are all required to make a decision on the basis of solid and scientific evidence from reliable data and information. The mission of the Biostatistics Division is to contribute to providing the best evidence and to the improvement of clinical practice and public health through the development and application of statistical methods. The Biostatistics Division is expanding on its independent and collaborative research in a range of areas, including prevention and policy recommendations/proposals, as well as treatment development. In addition, we have made a particular effort to build effective relationships with the investigators at the NCC Research Institute and the Institute of Cancer Control. Collaborative projects have been launched, including the evaluation and implementation of medical technology with artificial intelligence, and one of them received approval under the Pharmaceutical and Medical Device Act. Collaboration on epidemiologic studies has motivated us to develop statistical methods to get deeper insight into etiological mechanisms or public health impact. We are working on promoting a cooperative framework with outside experts in statistics/biostatistics. We are opening up a new methodological research area in which a mathematical approach will serve as a solid basis.
3. Bioethics Division
The Bioethics Division provides education and training on issues related to research ethics and conflicts of interest (COI), mainly in relation to the protection of human subjects, including ethical guidelines for life sciences and medical research involving human subjects, the Pharmaceutical Machine Law, the Clinical Research Law, laws related to regenerative medicine and the Personal Information Protection Law, and the operation of various ethical review committees for human subject research. The following are some of the activities.
-
The National Cancer Center’s “Training for new
employees” (delivered online in April).
Training on research ethics in the National Cancer Center’s ‘Clinical Research Seminar’ (June 2, September 16, October 7, November 4 and January 14 2022). - Training for Ethical Review Committee and Clinical Trial Review Committee members in 2021 (January 22 2022).
In addition to the provision of these education and training programs, the Bioethics and Medical Law Section and the COI Management Section, each of which is located in this division, carry out the following activities.
1) Bioethics and Healthcare Law Section
The Office of Bioethics and Healthcare Law provides research ethics consultation services to researchers, research assistants and research ethics review committee members at various stages from the planning of research to its publication. Unlike ethics review, research ethics consultation is non-binding advice and deals with other ethical issues beyond regulatory compliance.
FY 2021 results: 179 consultations were received in the year as a whole. 57 were from researchers and others at the Tsukiji campus and 122 were from researchers and others at the Kashiwa campus. There was one case relathe Clinical Research Act, 167 cases related to ethical guidelines and 11 cases related to other matters.ted to
(Future Prospects)
We will continue to provide and enhance our research ethics consultation services. In particular, in FY 2022, the Ethical Guidelines for Life Sciences and Medical Research Involving Human Subjects were revised following the past two years’ amendments to the Personal Information Protection Law, and we will strive to support the smooth implementation of research based on the new guidelines from an ethical perspective. Other research support activities related to the Center as a whole, in addition to consultations with individual researchers, will be continued as necessary.
2) COI Management Section
Our section serves as the COI management committee secretariat to manage NCC researchers’ COI and to respond to inquiries. The subjects of COI management are physician-initiated clinical trials, and some of the clinical research conducted under the Ethical Guidelines for Life Sciences and Medical Research Involving Human Subjects. We recommend that researchers involved in research projects funded by national ministries or authorities disclose their COI regardless of whether they are subject to COI review. Furthermore, we manage the COI of the members of the research ethics committee and institutional review board. We also handle the COI management required by the Clinical Trials Act.
FY 2021 results: The COI management committee reviewed 140 physician-initiated clinical trials (2856 researchers) and 193 clinical research projects conducted under the Ethical Guidelines for Life Sciences and Medical Research Involving Human Subjects (1918 researchers). We responded to 10 inquiries from researchers or staff. Our committee reviewed 29 members of the research ethics committee and 27 members of the institutional review board. Furthermore, we verified 113 COI disclosure forms pursuant to the Clinical Trials Act.
(Future Prospects)
We continue to improve the structure of COI review and management. In FY 2022, we will strive to develop the institutional COI management policy. Furthermore, we will complete the COI Declaration Management System to verify the researchers’ COI disclosure required by the Clinical Trials Act.
