Annual Report 2021
Division of Cancer Immunology
Hiroyoshi Nishikawa, Yuka Maeda, Keisuke Watanabe, Hitomi Nishinakamura, Kota Itahashi, Priya Saju, Kana Kusaba, Kenta Nakama, Ai Sasaki, Takeru Naito, Yuki Ishige, Sakthisri Krishnamurthy, Prakhongcheep Ornjira, Nanae Shimoyama
Introduction
Cancer immunotherapies such as immune checkpoint inhibitors (ICIs), chimeric antigen receptor T (CAR-T) cells or T- cell receptor T (TCR-T) cell therapies have shown significant efficacy in some patients. However, not a few patients experience relapse or eventually develop resistant disease. Cancers often create a complex immune suppressive environment that can impair the host immune reaction, and thereby impair the efficacy of ICIs or adoptive transferred anti-tumor T cells. Therefore, there is an urgent demand for elucidating the mechanisms of resistance and identifying precise biomarkers to predict efficacy in order to make immunotherapies a much more effective treatment.
The Team and What We Do
We have been intensively collecting clinical samples from cancer patients treated with immunotherapies, and investigating the mechanisms of cancer immunoreactions using multi-omics analysis, including flow cytometry, mass cytometry, imaging mass cytometry, single-cell transcriptome analysis, proteomics, and metabolomics (Figure 1). We are also developing first-in- class gene-modified cell-based immunotherapies using CAR-T cell or TCR-T cell platforms targeting the above mechanisms of resistance.
Figure 1. Investigation of the dynamic immune state in cancer
patients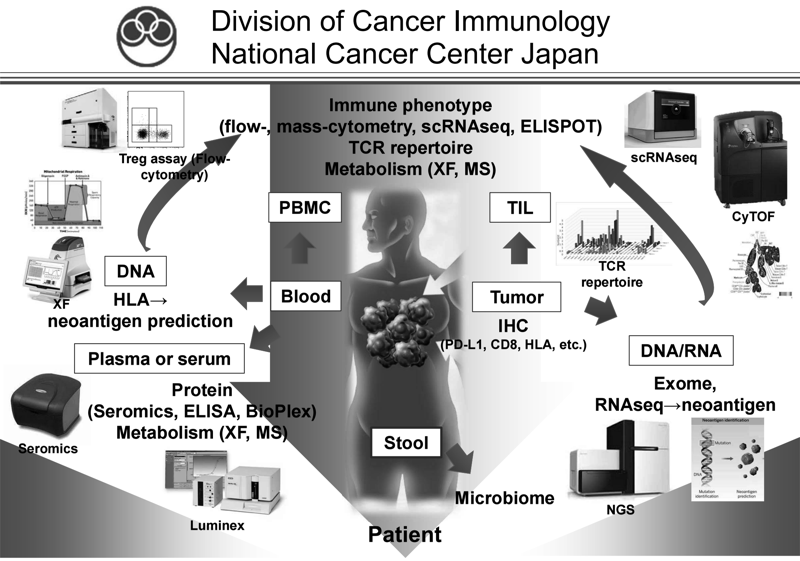

Research activities
We have identified multiple mechanisms that impair the efficacy of cancer immunotherapies using patient-derived clinical samples pre- and post-treatment with ICIs. In addition, we have revealed metabolic features of regulatory T cells (Tregs) that can survive in a low-nutrient tumor microenvironment and suppress the host immune reaction to the tumors. Interestingly, some tumors create metabolic features that metabolically support Treg survival in the TME to evade the host immune system. For further analysis of the interaction of immune cells with tumors and the TME, we have established mouse models that enable three-dimensional kinetic analysis of immune cells by rendering mouse tissues transparent (Figure 2). Using this system, we have started to elucidate the kinetics of immune cells during immunotherapies. We are also developing novel CAR-T cells that overcome multiple immunosuppressive factors, to improve the treatment outcome of adoptive cell therapies in solid tumors. Some of them are undergoing pre-clinical testing aiming for first-in-human trials.
Figure 2. Rendering mouse tissues transparent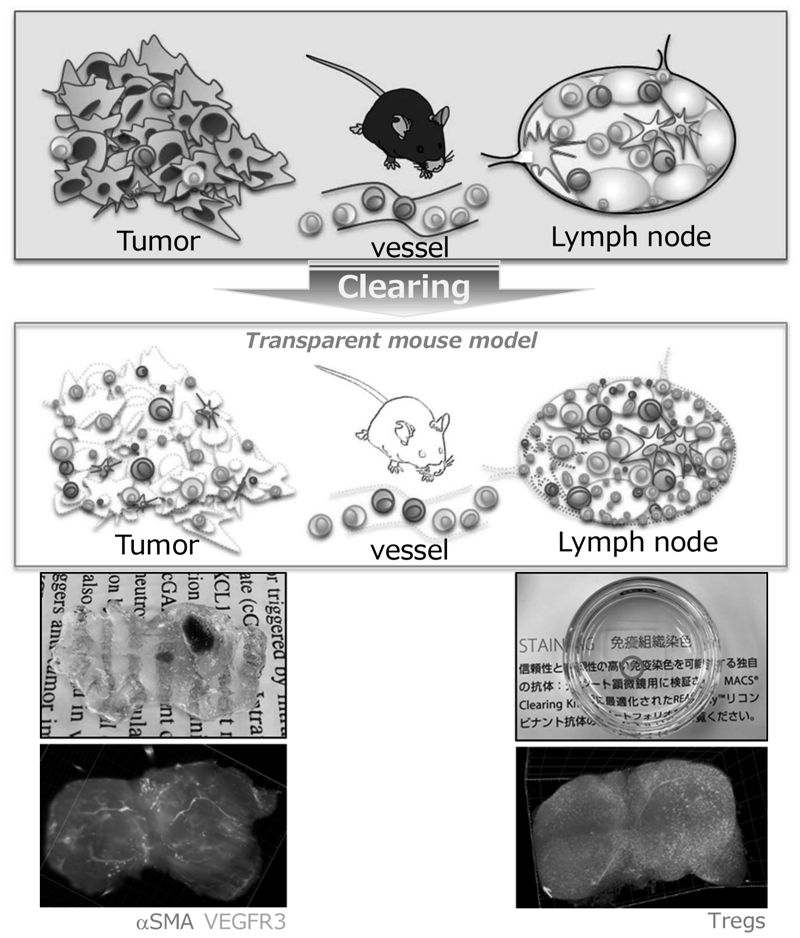

Clinical trials
We have collaborated with several pharmaceutical companies in their clinical trials to elucidate predictive biomarkers and immune status in the tumor microenvironment using our multi-omics immune analysis system.
Education
Our division is hosting graduate students from universities with partnership agreements, and young residents from the National Cancer Center Hospital for training in translational research. We are strongly supporting their career development, and many alumni are leading the immunology field in a variety of posts in clinics, academia, or paratheatrical companies.
Future Prospects
We will further investigate the mechanisms involved in tumor immune suppression or tumor immune evasion in various cancers with a broad view including areas such as immunology and genetics, using new approaches including special and dynamic analysis of the transcriptome or immune cell phenotypes, and transparent mouse tissue models. Our findings will lead to drug discoveries to overcome resistance and to the identification of biomarkers for immune therapies.
List of papers published in 2021
Journal
1. Aokage K, Tsuboi M, Zenke Y, Horinouchi H, Nakamura N, Ishikura S, Nishikawa H, Kumagai S, Koyama S, Kanato K, Kataoka T, Wakabayashi M, Fukutani M, Fukuda H, Ohe Y, Watanabe SI. Study protocol for JCOG1807C (DEEP OCEAN): a interventional prospective trial to evaluate the efficacy and safety of durvalumab before and after operation or durvalumab as maintenance therapy after chemoradiotherapy against superior sulcus non-small cell lung cancer. Japanese journal of clinical oncology, 52:383-387, 2022
2. Kumagai S, Koyama S, Itahashi K, Tanegashima T, Lin YT, Togashi Y, Kamada T, Irie T, Okumura G, Kono H, Ito D, Fujii R, Watanabe S, Sai A, Fukuoka S, Sugiyama E, Watanabe G, Owari T, Nishinakamura H, Sugiyama D, Maeda Y, Kawazoe A, Yukami H, Chida K, Ohara Y, Yoshida T, Shinno Y, Takeyasu Y, Shirasawa M, Nakama K, Aokage K, Suzuki J, Ishii G, Kuwata T, Sakamoto N, Kawazu M, Ueno T, Mori T, Yamazaki N, Tsuboi M, Yatabe Y, Kinoshita T, Doi T, Shitara K, Mano H, Nishikawa H. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer cell, 40:201-218.e9, 2022
3. Hasegawa H, Shitara K, Takiguchi S, Takiguchi N, Ito S, Kochi M, Horinouchi H, Kinoshita T, Yoshikawa T, Muro K, Nishikawa H, Suna H, Kodera Y. A multicenter, open-label, single-arm phase I trial of neoadjuvant nivolumab monotherapy for resectable gastric cancer. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association, 25:619-628, 2022
4. Bando H, Tsukada Y, Inamori K, Togashi Y, Koyama S, Kotani D, Fukuoka S, Yuki S, Komatsu Y, Homma S, Taketomi A, Uemura M, Kato T, Fukui M, Wakabayashi M, Nakamura N, Kojima M, Kawachi H, Kirsch R, Yoshida T, Suzuki Y, Sato A, Nishikawa H, Ito M, Yoshino T. Preoperative Chemoradiotherapy plus Nivolumab before Surgery in Patients with Microsatellite Stable and Microsatellite Instability-High Locally Advanced Rectal Cancer. Clinical cancer research: an official journal of the American Association for Cancer Research, 28:1136-1146, 2022
5. Kawazu M, Ueno T, Saeki K, Sax N, Togashi Y, Kanaseki T, Chida K, Kishigami F, Sato K, Kojima S, Otsuka M, Kawazoe A, Nishinakamura H, Yuka M, Yamamoto Y, Yamashita K, Inoue S, Tanegashima T, Matsubara D, Tane K, Tanaka Y, Iinuma H, Hashiguchi Y, Hazama S, Khor SS, Tokunaga K, Tsuboi M, Niki T, Eto M, Shitara K, Torigoe T, Ishihara S, Aburatani H, Haeno H, Nishikawa H, Mano H. HLA Class I Analysis Provides Insight Into the Genetic and Epigenetic Background of Immune Evasion in Colorectal Cancer With High Microsatellite Instability. Gastroenterology, 162:799-812, 2022
6. Nagasaki J, Inozume T, Sax N, Ariyasu R, Ishikawa M, Yamashita K, Kawazu M, Ueno T, Irie T, Tanji E, Morinaga T, Honobe A, Ohnuma T, Yoshino M, Iwata T, Kawase K, Sasaki K, Hanazawa T, Kochin V, Kawamura T, Matsue H, Hino M, Mano H, Suzuki Y, Nishikawa H, Togashi Y. PD-1 blockade therapy promotes infiltration of tumor-attacking exhausted T cell clonotypes. Cell reports, 38:110331, 2022
7. Miyai Y, Sugiyama D, Hase T, Asai N, Taki T, Nishida K, Fukui T, Chen-Yoshikawa TF, Kobayashi H, Mii S, Shiraki Y, Hasegawa Y, Nishikawa H, Ando Y, Takahashi M, Enomoto A. Meflin-positive cancer-associated fibroblasts enhance tumor response to immune checkpoint blockade. Life science alliance, 5:2022
8. Kawazoe A, Itahashi K, Yamamoto N, Kotani D, Kuboki Y, Taniguchi H, Harano K, Naito Y, Suzuki M, Fukutani M, Higuchi T, Ikeno T, Wakabayashi M, Sato A, Koyama S, Nishikawa H, Shitara K. TAS-116 (Pimitespib), an Oral HSP90 Inhibitor, in Combination with Nivolumab in Patients with Colorectal Cancer and Other Solid Tumors: An Open-Label, Dose-Finding, and Expansion Phase Ib Trial (EPOC1704). Clinical cancer research: an official journal of the American Association for Cancer Research, 27:6709-6715, 2021
9. Izumi H, Matsumoto S, Liu J, Tanaka K, Mori S, Hayashi K, Kumagai S, Shibata Y, Hayashida T, Watanabe K, Fukuhara T, Ikeda T, Yoh K, Kato T, Nishino K, Nakamura A, Nakachi I, Kuyama S, Furuya N, Sakakibara-Konishi J, Okamoto I, Taima K, Ebi N, Daga H, Yamasaki A, Kodani M, Udagawa H, Kirita K, Zenke Y, Nosaki K, Sugiyama E, Sakai T, Nakai T, Ishii G, Niho S, Ohtsu A, Kobayashi SS, Goto K. The CLIP1-LTK fusion is an oncogenic driver in non-small-cell lung cancer. Nature, 600:319-323, 2021
10. Kashima Y, Shibahara D, Suzuki A, Muto K, Kobayashi IS, Plotnick D, Udagawa H, Izumi H, Shibata Y, Tanaka K, Fujii M, Ohashi A, Seki M, Goto K, Tsuchihara K, Suzuki Y, Kobayashi SS. Single-Cell Analyses Reveal Diverse Mechanisms of Resistance to EGFR Tyrosine Kinase Inhibitors in Lung Cancer. Cancer research, 81:4835-4848, 2021
11. Isoyama S, Mori S, Sugiyama D, Kojima Y, Tada Y, Shitara K, Hinohara K, Dan S, Nishikawa H. Cancer immunotherapy with PI3K and PD-1 dual-blockade via optimal modulation of T cell activation signal. Journal for immunotherapy of cancer, 9:2021
12. Maeda Y, Wada H, Sugiyama D, Saito T, Irie T, Itahashi K, Minoura K, Suzuki S, Kojima T, Kakimi K, Nakajima J, Funakoshi T, Iida S, Oka M, Shimamura T, Doi T, Doki Y, Nakayama E, Ueda R, Nishikawa H. Depletion of central memory CD8+ T cells might impede the antitumor therapeutic effect of Mogamulizumab. Nature communications, 12:7280, 2021
13. Fujioka Y, Sugiyama D, Matsumura I, Minami Y, Miura M, Atsuta Y, Ohtake S, Kiyoi H, Miyazaki Y, Nishikawa H, Takahashi N. Regulatory T Cell as a Biomarker of Treatment-Free Remission in Patients with Chronic Myeloid Leukemia. Cancers, 13:2021
14. Takeuchi Y, Tanegashima T, Sato E, Irie T, Sai A, Itahashi K, Kumagai S, Tada Y, Togashi Y, Koyama S, Akbay EA, Karasaki T, Kataoka K, Funaki S, Shintani Y, Nagatomo I, Kida H, Ishii G, Miyoshi T, Aokage K, Kakimi K, Ogawa S, Okumura M, Eto M, Kumanogoh A, Tsuboi M, Nishikawa H. Highly immunogenic cancer cells require activation of the WNT pathway for immunological escape. Science immunology, 6:eabc6424, 2021
15. Kawashima S, Inozume T, Kawazu M, Ueno T, Nagasaki J, Tanji E, Honobe A, Ohnuma T, Kawamura T, Umeda Y, Nakamura Y, Kawasaki T, Kiniwa Y, Yamasaki O, Fukushima S, Ikehara Y, Mano H, Suzuki Y, Nishikawa H, Matsue H, Togashi Y. TIGIT/CD155 axis mediates resistance to immunotherapy in patients with melanoma with the inflamed tumor microenvironment. Journal for immunotherapy of cancer, 9:2021
16. Watanabe K, Nishikawa H. Engineering strategies for broad application of TCR-T- and CAR-T-cell therapies. International immunology, 33:551-562, 2021
17. Nishikawa H, Koyama S. Mechanisms of regulatory T cell infiltration in tumors: implications for innovative immune precision therapies. Journal for immunotherapy of cancer, 9:2021
18. Satoh K, Kobayashi Y, Fujimaki K, Hayashi S, Ishida S, Sugiyama D, Sato T, Lim K, Miyamoto M, Kozuma S, Kadokura M, Wakita K, Hata M, Hirahara K, Amano M, Watanabe I, Okamoto A, Tuettenberg A, Jonuleit H, Tanemura A, Maruyama S, Agatsuma T, Wada T, Nishikawa H. Novel anti-GARP antibody DS-1055a augments anti-tumor immunity by depleting highly suppressive GARP+ regulatory T cells. International immunology, 33:435-446, 2021
19. Yasuda Y, Iwama S, Sugiyama D, Okuji T, Kobayashi T, Ito M, Okada N, Enomoto A, Ito S, Yan Y, Sugiyama M, Onoue T, Tsunekawa T, Ito Y, Takagi H, Hagiwara D, Goto M, Suga H, Banno R, Takahashi M, Nishikawa H, Arima H. CD4+ T cells are essential for the development of destructive thyroiditis induced by anti-PD-1 antibody in thyroglobulin-immunized mice. Science translational medicine, 13:2021
20. Kobayashi T, Iwama S, Sugiyama D, Yasuda Y, Okuji T, Ito M, Ito S, Sugiyama M, Onoue T, Takagi H, Hagiwara D, Ito Y, Suga H, Banno R, Nishikawa H, Arima H. Anti-pituitary antibodies and susceptible human leukocyte antigen alleles as predictive biomarkers for pituitary dysfunction induced by immune checkpoint inhibitors. Journal for immunotherapy of cancer, 9:2021
21. Kitahara M, Shigeno Y, Shime M, Matsumoto Y, Nakamura S, Motooka D, Fukuoka S, Nishikawa H, Benno Y. Vescimonas gen. nov., Vescimonas coprocola sp. nov., Vescimonas fastidiosa sp. nov., Pusillimonas gen. nov. and Pusillimonas faecalis sp. nov. isolated from human faeces. International journal of systematic and evolutionary microbiology, 71:2021
22. Kelkka T, Savola P, Bhattacharya D, Huuhtanen J, Lönnberg T, Kankainen M, Paalanen K, Tyster M, Lönnberg M, Ellonen P, Smolander J, Eldfors S, Yadav B, Khan S, Koivuniemi R, Sjöwall C, Elo LL, Lähdesmäki H, Maeda Y, Nishikawa H, Leirisalo-Repo M, Sokka-Isler T, Mustjoki S . Corrigendum: Adult-Onset Anti-Citrullinated Peptide Antibody-Negative Destructive Rheumatoid Arthritis Is Characterized by a Disease-Specific CD8+ T Lymphocyte Signature. Frontiers in immunology, 12:710831, 2021
23. Saito T, Kurose K, Kojima T, Funakoshi T, Sato E, Nishikawa H, Nakajima J, Seto Y, Kakimi K, Iida S, Doki Y, Oka M, Ueda R, Wada H. Phase Ib study on the humanized anti-CCR4 antibody, KW-0761, in advanced solid tumors. Nagoya journal of medical science, 83:827-840, 2021
24. Good CR, Aznar MA, Kuramitsu S, Samareh P, Agarwal S, Donahue G, Ishiyama K, Wellhausen N, Rennels AK, Ma Y, Tian L, Guedan S, Alexander KA, Zhang Z, Rommel PC, Singh N, Glastad KM, Richardson MW, Watanabe K, Tanyi JL, O'Hara MH, Ruella M, Lacey SF, Moon EK, Schuster SJ, Albelda SM, Lanier LL, Young RM, Berger SL, June CH. An NK-like CAR T cell transition in CAR T cell dysfunction. Cell, 184:6081-6100.e26, 2021
25. Tanaka K, Yu HA, Yang S, Han S, Selcuklu SD, Kim K, Ramani S, Ganesan YT, Moyer A, Sinha S, Xie Y, Ishizawa K, Osmanbeyoglu HU, Lyu Y, Roper N, Guha U, Rudin CM, Kris MG, Hsieh JJ, Cheng EH. Targeting Aurora B kinase prevents and overcomes resistance to EGFR inhibitors in lung cancer by enhancing BIM- and PUMA-mediated apoptosis. Cancer cell, 39:1245-1261.e6, 2021
