Annual Report 2022
Section of Knowledge Integration
Shinji Kohsaka, Katsuyuki Ikarashi, Kayoko Tao, Taro Shibata, Yasushi Goto, Kazuya Tsuchihara
Introduction
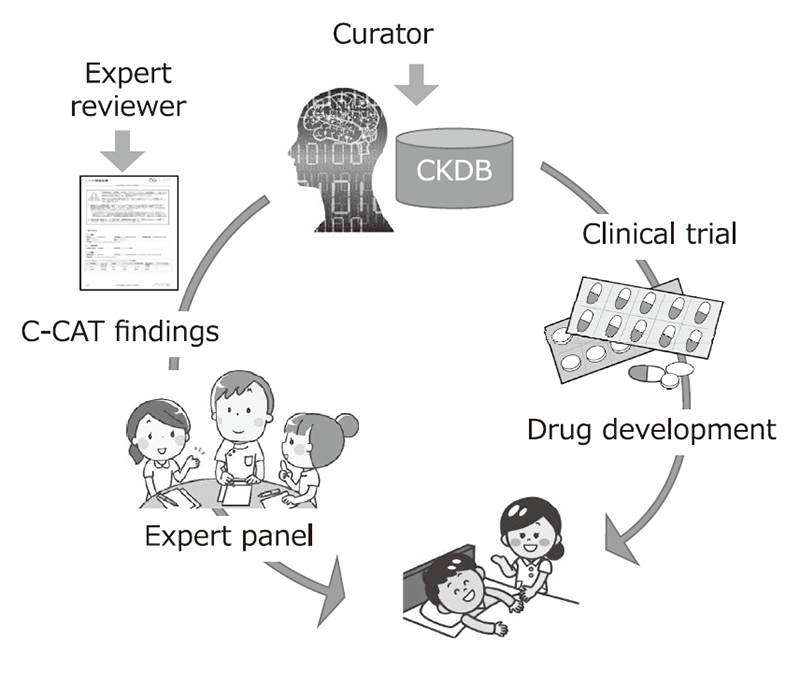
The Section of Knowledge Integration is constructing the Cancer Knowledge Database (CKDB) to control the quality and advance the genomic diagnosis of cancer. The section coordinates with the information repository system developed by the Section of Cancer Genomics Repository and optimizes the CKDB for Japan to support the expert panels at core hospitals for cancer genomic medicine.
The Team and What We Do
- Development of Cancer Knowledge Database
- Providing the C-CAT findings
The CKDB was developed and maintained to improve the quality of cancer genome testing. A curator team consisting of 26 clinical oncologists and bioinformaticians was organized to maintain the system, updating data from clinical trials, medicines and marker evidence monthly.
We have provided expert panel C-CAT findings, a report summary of clinical trials, medicines and marker evidence to annotate clinical significance of variants found by cancer panel testing performed by clinical laboratories. We have also established and maintained a management system to control the quality of C-CAT findings by standardizing the review process by expert reviewers, who are equivalent to M.D. or Ph.D. level.
Research Activities
The CKDB was developed by collecting, standardizing and integrating the information of marker evidence, anti-cancer therapies and clinical trials. Using the CKDB, we constructed a system to generate C-CAT findings. We also developed the CKDB-portal to search, query and curate the knowledge stored in CKDB. As of March 2023, information on 774 clinical trials, 1,510 medicines, and 29,866 items of marker evidence are stored in CKDB. We signed non-disclosure agreements with six pharmaceutical companies to receive detailed information from 126 clinical trials between April 2022 and March 2023. A total of 20,238 C-CAT findings were provided to expert panels of core hospitals between April 2022 and March 2023.
Clinical Trials
The Japan Clinical Oncology Group (JCOG)-Brain Tumor Study Group was organized in 2002 and multi-institutional randomized controlled trials are ongoing. A “Randomized Phase III study for unresectable WHO Grade II astrocytoma with radiotherapy alone or chemoradiotherapy with temozolomide (JCOG1303)”, a Phase III randomized study for grade III gliomas (JCOG1016), and Phase III study for glioblastoma (JCOG1308, JCOG1703 and JCOG1910) are underway. These studies, under the surveillance of the JCOG, aim to set a standard protocol for treating malignant brain tumor patients. Moreover, a proper methodology for performing randomized studies will be established in the field of neuro-oncology.
An investigator-initiated phase II clinical trial of eribulin targeting TERT in patients with recurrent glioblastomas and a phase II study of temozolomide and metformin for newly-diagnosed glioblastoma are also ongoing under AMED grants.
Education
Our department plays the pivotal role of the JCOG-Brain Tumor Study Group and the brain tumor registry of Japan, and we conducted many clinical trials and ran the brain tumor registry. We educate many neurosurgeons and oncologists about surgical techniques of awake craniotomy and intraoperative MRI and the effective usage and adverse effects of many chemotherapeutic agents for malignant brain tumors.
Future Prospects
Malignant brain tumors, especially glioblastoma still have the worse prognosis among all cancers. We continually strive to defeat these brain cancers through a variety of clinical work and research.
Figure 1. Development of CKDB to improve the quality of genome medicine