Annual Report 2022
Department of Gastroenterology and Gastrointestinal Oncology
Kouhei Shitara, Takashi Kojima, Hideaki Bando, Yasutoshi Kuboki, Akihito Kawazoe, Nozomu Fuse, Takayuki Yoshino, Daisuke Kotani, Yoshiaki Nakamura, Saori Mishima, Ayumu Yoshikawa
Introduction
In 2022, approximately 800 patients with gastrointestinal (GI) cancer were treated by staff oncologists and skilled residents in the Department of GI Oncology, which focuses on the optimal chemotherapy with or without radiation for the treatment of GI cancers.
The Team and What We Do
Inter-divisional tumor board conferences with the Surgical/Radiation Oncology Divisions are held regularly to review the current treatment for each patient and to discuss further treatment strategies. Our activities for each type of GI cancer in 2022 are shown in Table 1. There are ongoing clinical trials, which consist of 73 phase I trials, including global first-in-class (FIC), first-in-human (FIH), and investigational new drugs (INDs), 69 phase II/III clinical trials to approve the INDs, and 17 investigator-initiated clinical trials (IITs). Additionally, young skilled residents in their early 30s have become principal investigators for several IITs.
Table 1. Number of new patients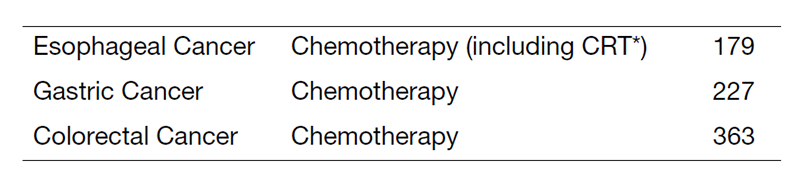
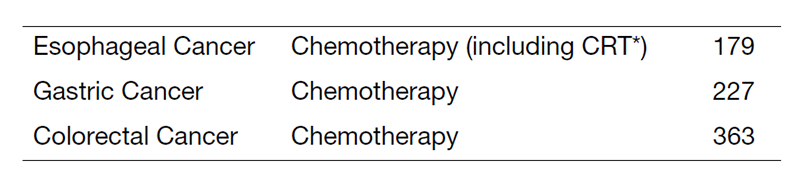
Activities
Esophageal Cancer (EC)
The results of a phase I trial that evaluates the safety and efficacy of an oncolytic virus with pembrolizumab and a phase Ib/II study that investigates the safety, efficacy, and proof-of-concept (POC) of Anti PD-L1 antibody monotherapy following radical chemoradiotherapy were reported at AACR2023 and ESMO 2022, respectively. An observational study to clarify the clinical utility of detecting MRD and monitoring using ctDNA analysis in patients with gastrointestinal cancer who plan to undergo curative treatment is ongoing, and the interim analysis was reported at ASCO 2023. Furthermore, we contribute to ongoing international global studies including a phase III trial of lenvatinib and pembrolizumab in combination with chemotherapy for advanced esophageal squamous cell carcinoma.
Gastric Cancer (GC)
We reported several studies to clarify the immune profiles or multiple biomarker expression in gastric cancer as collaborations with the division of cancer immunology, pathological department and pharmaceutical company. Two global phase III studies (LEAP-015 and INTEGRATE2b) are ongoing based on our investigator-initiated trials of lenvatinib plus pembrolizumab or regorafenib plus nivolumab for gastric cancer. Furthermore, we completed enrollment for a phase II study of first-line chemotherapy and regorafenib plus nivolumab. The results from the phase I trial of photoimmunotherapy plus nivolumab for patients with EGFR expressing gastric cancer were reported at DDW 2023. Furthermore, based on an ongoing phase II trial of neoadjuvant treatment with chemotherapy and lenvatinib plus pembrolizumab, a new trial is currently planned to evaluate a combination with chemoradiotherapy for localized gastroesophageal cancer. A phase II study of neoadjuvant trastuzumab deruxtecan is ongoing with completed enrollment for the T-DXd monotherapy cohort and new cohort to evaluate combination with chemotherapy and immunotherapy will be incorporated. As a new concept, an investigator-initiated trial will be started to combine sulfasalazine and oxyfedrine to induce ferroptosis and cancer cell death. Our department contributed to several international global studies including a phase III trial of zolbetuximab plus chemotherapy (SPOTLIGHT) for gastric cancer and published the results in major journals such as the Lancet as the first/lead author.
Colorectal Cancer (CRC)
We have been conducting our initiative SCRUM-Japan platform, which is a nationwide cancer genome screening system using tissue/plasma-based next-generation sequencing (MONSTAR-SCREEN-2, GOZILA) and whole exome/whole transcriptome sequencing for metastatic solid tumors. Utilizing these screening systems, the umbrella type of IITs for patients with metastatic CRC or solid tumors with BRAF non-V600E mutation, MET amplification, and MSI-high resectable rectal cancer are ongoing. Further, we have launched IITs in resectable colorectal cancer (CIRCULATE-Japan project) to stratify them according to recurrence risk based on the Signatera assay for detecting minimal residual disease. Most recently, our department contributed to several international global studies including a phase III trial of lenvatinib and pembrolizumab (LEAP-017) for colorectal cancer and presented the results in ESMO GI 2023 as the first/lead author.
Education
Our residents learn the latest evidence-based medicine and pragmatically apply this knowledge to enhance care for patients with GI cancers, and eventually qualify as comprehensive GI oncologists through daily practice and direct training from our staff. Accordingly, our staff actively provide numerous valuable opportunities to polish their chemotherapy skills, especially in collaboration with the Department of Experimental Therapeutics, as well as diagnostic and therapeutic endoscopy skills in collaboration with the Department of Digestive Endoscopy. We regularly hold tumor board meetings and frequently conduct numerous face-to-face meetings with experts in different fields. We instruct them on how to conduct valuable clinical trials, help them attend international academic conferences, and explain the best way to present at academic meetings as well as work on many high-impact articles in scholarly journals. To date, our department has helped many residents to become ‘true’ skilled GI oncologists who play major roles at leading cancer centers nationwide.
Future Prospects
We continue to provide the best treatment for patients with cancer and the best education for residents, and aim to perform the following activities:
1) Provide more of the latest, cutting-edge medicine to patients with cancer and to foster the next generation of skilled GI oncologists.
2) Achieve medical innovation in Japan and play a leading role in the clinical development of INDs by contributing to various clinical trials including FIC, FIH early trials, IITs with proof-of-concept, and international clinical trials.
3) Establish global research networks with cutting-edge researchers to enhance our research activities.
List of papers published in 2022
Journal
1. Udagawa H, Takahashi S, Hirao M, Tahara M, Iwasa S, Sato Y, Hamakawa T, Shitara K, Horinouchi H, Chin K, Masuda N, Suzuki T, Okumura S, Takase T, Nagai R, Yonemori K. Liposomal eribulin for advanced adenoid cystic carcinoma, gastric cancer, esophageal cancer, and small cell lung cancer. Cancer medicine, 12:1269-1278, 2023
2. Doi T, Shitara K, Kojima T, Kuboki Y, Matsubara N, Bando H, Yoh K, Naito Y, Hirai H, Kurokawa Y, Kato T, Morizane C. Phase I study of the irreversible fibroblast growth factor receptor 1-4 inhibitor futibatinib in Japanese patients with advanced solid tumors. Cancer science, 114:574-585, 2023
3. Shitara K, Hirao M, Iwasa S, Oshima T, Komatsu Y, Kawazoe A, Sato Y, Hamakawa T, Yonemori K, Machida N, Yuki S, Suzuki T, Okumura S, Takase T, Semba T, Zimmermann B, Teng A, Yamaguchi K. Phase I Study of the Liposomal Formulation of Eribulin (E7389-LF): Results from the Advanced Gastric Cancer Expansion Cohort. Clinical cancer research, 29:1460-1467, 2023
4. Matsubara Y, Masuishi T, Ogata T, Nakazawa T, Kato K, Nozawa K, Narita Y, Honda K, Bando H, Taniguchi H, Kadowaki S, Ando M, Tajika M, Muro K. Impact of omitting fluorouracil from FOLFIRI plus bevacizumab as second-line chemotherapy for patients with metastatic colorectal cancer. Journal of cancer research and clinical oncology, 149:1123-1129, 2023
5. Liu T, Bai Y, Lin X, Li W, Wang J, Zhang X, Pan H, Bai C, Bai L, Cheng Y, Zhang J, Zhong H, Ba Y, Hu W, Xu R, Guo W, Qin S, Yang N, Lu J, Shitara K, Lei M, Li M, Bao N, Chen T, Shen L. First-line nivolumab plus chemotherapy vs chemotherapy in patients with advanced gastric, gastroesophageal junction and esophageal adenocarcinoma: CheckMate 649 Chinese subgroup analysis. International journal of cancer, 152:749-760, 2023
6. Gallois C, Shi Q, Meyers JP, Iveson T, Alberts SR, de Gramont A, Sobrero AF, Haller DG, Oki E, Shields AF, Goldberg RM, Kerr R, Lonardi S, Yothers G, Kelly C, Boukovinas I, Labianca R, Sinicrope FA, Souglakos I, Yoshino T, Meyerhardt JA, André T, Papamichael D, Taieb J. Prognostic Impact of Early Treatment and Oxaliplatin Discontinuation in Patients With Stage III Colon Cancer: An ACCENT/IDEA Pooled Analysis of 11 Adjuvant Trials. Journal of clinical oncology, 41:803-815, 2023
7. Watanabe J, Terazawa T, Yamane S, Kazama H, Uetake H, Yoshino T. Aflibercept with FOLFIRI in Japanese patients with metastatic colorectal cancer: results of a post-marketing surveillance. International journal of clinical oncology, 28:130-138, 2023
8. Sakata H, Murase M, Kato T, Yamaguchi K, Sugihara K, Suzuki S, Yoshino T. Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer: an early post-marketing phase vigilance study. International journal of clinical oncology, 28:139-144, 2023
9. Yoshino T, Andre T, Kim TW, Yong WP, Shiu KK, Jensen BV, Jensen LH, Punt CJA, Smith D, Garcia-Carbonero R, Alcaide-Garcia J, Gibbs P, de la Fouchardiere C, Rivera F, Elez E, Le DT, Adachi N, Fogelman D, Marinello P, Diaz LA Jr. Pembrolizumab in Asian patients with microsatellite-instability-high/mismatch-repair-deficient colorectal cancer. Cancer science, 114:1026-1036, 2023
10. Sakamoto Y, Bando H, Nakamura Y, Hasegawa H, Kuwaki T, Okamoto W, Taniguchi H, Aoyagi Y, Miki I, Uchigata H, Kuramoto N, Fuse N, Yoshino T, Ohtsu A. Trajectory for the Regulatory Approval of a Combination of Pertuzumab Plus Trastuzumab for Pre-treated HER2-positive Metastatic Colorectal Cancer Using Real-world Data. Clinical colorectal cancer, 22:45-52, 2023
11. Yamaguchi K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, Chung HC, Kawakami H, Yabusaki H, Lee J, Shimoyama T, Lee KW, Saito K, Kawaguchi Y, Kamio T, Kojima A, Sugihara M, Shitara K. Trastuzumab Deruxtecan in Anti-Human Epidermal Growth Factor Receptor 2 Treatment-Naive Patients With Human Epidermal Growth Factor Receptor 2-Low Gastric or Gastroesophageal Junction Adenocarcinoma: Exploratory Cohort Results in a Phase II Trial. Journal of clinical oncology, 41:816-825, 2023
12. Vaghi C, Mauri G, Agostara AG, Patelli G, Pizzutilo EG, Nakamura Y, Yoshino T, Siena S, Sartore-Bianchi A. The predictive role of ERBB2 point mutations in metastatic colorectal cancer: A systematic review. Cancer treatment reviews, 112:102488, 2023
13. Margalit O, Harmsen WS, Shacham-Shmueli E, Voss MM, Boursi B, Wagner AD, Cohen R, Olswold CL, Saltz LB, Goldstein DA, Hurwitz H, Tebbutt NC, Kabbinavar FF, Adams RA, Chibaudel B, Grothey A, Yoshino T, Zalcberg J, de Gramont A, Shi Q, Lenz HJ. Evaluating sex as a predictive marker for response to bevacizumab in metastatic colorectal carcinoma: Pooled analysis of 3,369 patients in the ARCAD database. European journal of cancer (Oxford, England : 1990), 178:162-170, 2023
14. Shitara K, Kawazoe A, Hirakawa A, Nakanishi Y, Furuki S, Fukuda M, Ueno Y, Raizer J, Arozullah A. Phase 1 trial of zolbetuximab in Japanese patients with CLDN18.2+ gastric or gastroesophageal junction adenocarcinoma. Cancer science, 114:1606-1615, 2023
15. Shah MA, Yoshino T, Tebbutt NC, Grothey A, Tabernero J, Xu RH, Cervantes A, Oh SC, Yamaguchi K, Fakih M, Falcone A, Wu C, Chiu VK, Tomasek J, Bendell J, Fontaine M, Hitron M, Xu B, Taieb J, Van Cutsem E. Napabucasin Plus FOLFIRI in Patients With Previously Treated Metastatic Colorectal Cancer: Results From the Open-Label, Randomized Phase III CanStem303C Study. Clinical colorectal cancer, 22:100-110, 2023
16. Kanai M, Kawaguchi T, Kotaka M, Manaka D, Hasegawa J, Takagane A, Munemoto Y, Kato T, Eto T, Touyama T, Matsui T, Shinozaki K, Matsumoto S, Mizushima T, Mori M, Sakamoto J, Ohtsu A, Yoshino T, Saji S, Matsuda F. Poor association between dihydropyrimidine dehydrogenase (DPYD) genotype and fluoropyrimidine-induced toxicity in an Asian population. Cancer medicine, 12:7808-7814, 2023
17. Sato S, Nakamura Y, Oki E, Yoshino T. Molecular Residual Disease-guided Adjuvant Treatment in Resected Colorectal Cancer: Focus on CIRCULATE-Japan. Clinical colorectal cancer, 22:53-58, 2023
18. Kotani D, Nakamura Y, Fujisawa T, Bando H, Sakamoto N, Johns AL, Park K, Casolino R, Yoshino T, Biankin AV. ICGC-ARGO precision medicine: an update on targeted therapy based on longitudinal analysis of tumour heterogeneity and evolution in colorectal cancer. The Lancet. Oncology, 24:20-21, 2023
19. Bando H, Ohtsu A, Yoshino T. Therapeutic landscape and future direction of metastatic colorectal cancer. Nature reviews. Gastroenterology & hepatology, 20:306-322, 2023
20. Isaka Y, Sasaki A, Saito A, Motomura Y, Ando Y, Nakamura Y. Exceptional response to alectinib for duodenal carcinoma with ALK fusion: A case report and literature review. Frontiers in oncology, 12:1064944, 2023
21. Yoshino T, Taieb J, Kuboki Y, Pfeiffer P, Kumar A, Hochster HS. Trifluridine/tipiracil with or without bevacizumab in metastatic colorectal cancer: results of a systematic review and meta-analysis. Therapeutic advances in medical oncology, 15:17588359221146137, 2023
22. Ohba A, Morizane C, Ueno M, Kobayashi S, Kawamoto Y, Komatsu Y, Ikeda M, Sasaki M, Okano N, Furuse J, Hiraoka N, Yoshida H, Kuchiba A, Sadachi R, Nakamura K, Matsui N, Nakamura Y, Okamoto W, Yoshino T, Okusaka T. Multicenter phase II trial of trastuzumab deruxtecan for HER2-positive unresectable or recurrent biliary tract cancer: HERB trial. Future oncology (London, England), 18:2351-2360, 2022
23. Hasegawa H, Shitara K, Takiguchi S, Takiguchi N, Ito S, Kochi M, Horinouchi H, Kinoshita T, Yoshikawa T, Muro K, Nishikawa H, Suna H, Kodera Y. A multicenter, open-label, single-arm phase I trial of neoadjuvant nivolumab monotherapy for resectable gastric cancer. Gastric cancer, 25:619-628, 2022
24. Ito R, Nakamura Y, Sunakawa H, Fujiwara H, Hojo H, Nakamura N, Fujita T, Yano T, Daiko H, Akimoto T, Yoshino T, Kojima T. Tumor response and survival outcomes of salvage concurrent chemoradiotherapy with three-dimensional conformal radiotherapy and 5-fluorouracil/platinum-based chemotherapy for postoperative locoregional recurrence of esophageal squamous cell carcinoma. Esophagus, 19:645-652, 2022
25. Kikuchi K, Yamazaki N, Nozawa K, Fukuda H, Shibata T, Machida R, Hamaguchi T, Takashima A, Shoji H, Boku N, Takatsuka S, Takenouchi T, Nishina T, Yoshikawa S, Takahashi M, Hasegawa A, Kawazoe A, Masuishi T, Mizutani H, Kiyohara Y. Topical corticosteroid therapy for facial acneiform eruption due to EGFR inhibitors in metastatic colorectal cancer patients: a randomized controlled trial comparing starting with a very strong or a weak topical corticosteroid (FAEISS study, NCCH1512, colorectal part). Supportive care in cancer, 30:4497-4504, 2022
26. Mizukami T, Takahashi M, Sunakawa Y, Yuki S, Kagawa Y, Takashima A, Kato K, Hara H, Denda T, Yamamoto Y, Shiozawa M, Oki E, Okamoto W, Yoshino T, Eguchi Nakajima T. Genomic Landscape of Primary Tumor Site and Clinical Outcome for Patients with Metastatic Colorectal Cancer Receiving Standard-of-Care Chemotherapy. Targeted oncology, 17:343-353, 2022
27. Kajiwara T, Nishina T, Nakasya A, Yamashita N, Yamashita R, Nakamura Y, Shiozawa M, Yuki S, Taniguchi H, Hara H, Ohta T, Esaki T, Shinozaki E, Takashima A, Moriwaki T, Denda T, Ohtsubo K, Sunakawa Y, Horita Y, Kawakami H, Kato T, Satoh T, Ando K, Mizutani T, Yasui H, Goto M, Okuyama H, Yamazaki K, Yoshino T, Hyodo I. NOTCH gene alterations in metastatic colorectal cancer in the Nationwide Cancer Genome Screening Project in Japan (SCRUM-Japan GI-SCREEN). Journal of cancer research and clinical oncology, 148:2841-2854, 2022
28. Yamaguchi K, Minashi K, Sakai D, Nishina T, Omuro Y, Tsuda M, Iwagami S, Kawakami H, Esaki T, Sugimoto N, Oshima T, Kato K, Amagai K, Hosaka H, Komine K, Yasui H, Negoro Y, Ishido K, Tsushima T, Han S, Shiratori S, Takami T, Shitara K. Phase IIb study of pembrolizumab combined with S-1 + oxaliplatin or S-1 + cisplatin as first-line chemotherapy for gastric cancer. Cancer science, 113:2814-2827, 2022
29. Hino K, Nishina T, Kajiwara T, Bando H, Nakamura M, Kadowaki S, Minashi K, Yuki S, Ohta T, Hara H, Mizukami T, Moriwaki T, Ohtsubo K, Komoda M, Mitani S, Nagashima F, Kato K, Yamada T, Hasegawa H, Yamazaki K, Yoshino T, Hyodo I. Association of ERBB2 Copy Number and Gene Coalterations With Trastuzumab Efficacy and Resistance in Human Epidermal Growth Factor Receptor 2-Positive Esophagogastric and Gastric Cancer. JCO precision oncology, 6:e2200135, 2022
30. Kato T, Matsubara N, Shiota M, Eto M, Osawa T, Abe T, Shinohara N, Yasumizu Y, Tanaka N, Oya M, Nishimoto K, Hayashi T, Nakayama M, Kojima T, Namikawa K, Fujisawa T, Okano S, Hida E, Nakamura Y, Bando H, Yoshino T, Nonomura N. IMAGENE trial: multicenter, proof-of-concept, phase II study evaluating the efficacy and safety of combination therapy of niraparib with PD-1 inhibitor in solid cancer patients with homologous recombination repair genes mutation. BMC cancer, 22:1292, 2022
31. Imai M, Nakamura Y, Sunami K, Kage H, Komine K, Koyama T, Amano T, Ennishi D, Kanai M, Kenmotsu H, Maeda T, Morita S, Sakai D, Bando H, Makiyama A, Suzuki T, Hirata M, Kohsaka S, Tsuchihara K, Naito Y, Yoshino T. Expert panel consensus recommendations on the use of circulating tumor DNA assays for patients with advanced solid tumors. Cancer science, 113:3646-3656, 2022
32. Sunami K, Naito Y, Komine K, Amano T, Ennishi D, Imai M, Kage H, Kanai M, Kenmotsu H, Koyama T, Maeda T, Morita S, Sakai D, Kohsaka S, Tsuchihara K, Saigusa Y, Yoshino T. Chronological improvement in precision oncology implementation in Japan. Cancer science, 113:3995-4000, 2022
33. Naito Y, Sunami K, Kage H, Komine K, Amano T, Imai M, Koyama T, Ennishi D, Kanai M, Kenmotsu H, Maeda T, Morita S, Sakai D, Watanabe K, Shirota H, Kinoshita I, Yoshioka M, Mamesaya N, Ito M, Kohsaka S, Saigusa Y, Yamamoto K, Hirata M, Tsuchihara K, Yoshino T. Concordance Between Recommendations From Multidisciplinary Molecular Tumor Boards and Central Consensus for Cancer Treatment in Japan. JAMA network open, 5:e2245081, 2022
34. Adachi M, Aoyama N, Kojima M, Sakamoto N, Miyazaki S, Taki T, Watanabe R, Matsuura K, Kotani D, Kojima T, Fujita T, Tabuchi K, Ishii G, Sakashita S. The area of residual tumor predicts esophageal squamous cell carcinoma prognosis following neoadjuvant chemotherapy. Journal of cancer research and clinical oncology, 2022
35. Kotani D, Nakamura Y, Fujisawa T, Bando H, Sakamoto N, Johns AL, Park K, Casolino R, Yoshino T, Biankin AV. ICGC-ARGO precision medicine: targeted therapy according to longitudinal assessment of tumour heterogeneity in colorectal cancer. The Lancet. Oncology, 23:463-464, 2022
36. Sakai SA, Aoshima M, Sawada K, Horasawa S, Yoshikawa A, Fujisawa T, Kadowaki S, Denda T, Matsuhashi N, Yasui H, Goto M, Yamazaki K, Komatsu Y, Nakanishi R, Nakamura Y, Bando H, Hamaya Y, Kageyama SI, Yoshino T, Tsuchihara K, Yamashita R. Fecal microbiota in patients with a stoma decreases anaerobic bacteria and alters taxonomic and functional diversities. Frontiers in cellular and infection microbiology, 12:925444, 2022
37. Mizuno M, Chiba I, Mukohara T, Kondo M, Maruo K, Ohigashi T, Naruo M, Asano Y, Onishi T, Tanabe H, Muta R, Mishima S, Okano S, Yuda M, Hosono A, Ueda Y, Bando H, Itagaki H, Ferrans CE, Akimoto T. Effectiveness of an online support program to help female cancer patients manage their health and illness: Protocol for a randomized controlled trial. Contemporary clinical trials communications, 30:101035, 2022
38. Okunaka M, Kotani D, Demachi K, Fujiwara H, Sakashita S, Yoshino T, Fujita T, Kojima T. Significance of chemotherapy-free interval and tumor regression grade in patients with recurrent esophageal squamous cell carcinoma receiving chemotherapy with fluorouracil and platinum after esophagectomy following preoperative chemotherapy. Esophagus, 19:240-249, 2022
39. Sato D, Kadota T, Inaba A, Nishihara K, Takashima K, Nakajo K, Sawada K, Kotani D, Fujiwara H, Yoda Y, Kojima T, Fujita T, Fujii S, Yano T. Long-term clinical outcome after endoscopic resection of esophageal squamous cell carcinoma invading the muscularis mucosae without lymphovascular invasion. Gastrointestinal endoscopy, 95:634-641.e3, 2022
40. Sawada K, Kotani D, Yukami H, Mishima S, Fujiwara H, Kadota T, Nakajo K, Yoda Y, Nakamura M, Hojo H, Yano T, Fujita T, Kojima T. Definitive chemoradiotherapy has comparable survival outcomes to esophagectomy in patients with clinical T1N0M0 esophageal squamous cell carcinoma: real-world data. International journal of clinical oncology, 27:1279-1288, 2022
41. Harada T, Tsuji T, Ueno J, Koishihara Y, Konishi N, Hijikata N, Ishikawa A, Kotani D, Kojima T, Fujiwara H, Fujita T. Prognostic Impact of the Loss of Skeletal Muscle Mass During Neoadjuvant Chemotherapy on Older Patients with Esophageal Cancer. Annals of surgical oncology, 29:8131-8139, 2022
42. Harada T, Tsuji T, Ueno J, Koishihara Y, Konishi N, Hijikata N, Ishikawa A, Kotani D, Kojima T, Fujiwara H, Fujita T. ASO Visual Abstract: Prognostic Impact of the Loss of Skeletal Muscle Mass during Neoadjuvant Chemotherapy on Older Patients with Esophageal Cancer. Annals of surgical oncology, 29:8142-8143, 2022
43. Miyo M, Kato T, Nakamura Y, Taniguchi H, Takahashi Y, Ishii M, Okita K, Ando K, Yukami H, Mishima S, Yamazaki K, Kotaka M, Watanabe J, Oba K, Aleshin A, Billings PR, Rabinowitz M, Kotani D, Oki E, Takemasa I, Mori M, Yoshino T. DENEB: Development of new criteria for curability after local excision of pathological T1 colorectal cancer using liquid biopsy. Cancer science, 113:1531-1534, 2022
44. Goldberg RM, Adams R, Buyse M, Eng C, Grothey A, André T, Sobrero AF, Lichtman SM, Benson AB, Punt CJA, Maughan T, Burzykowski T, Sommeijer D, Saad ED, Shi Q, Coart E, Chibaudel B, Koopman M, Schmoll HJ, Yoshino T, Taieb J, Tebbutt NC, Zalcberg J, Tabernero J, Van Cutsem E, Matheson A, de Gramont A. Clinical Trial Endpoints in Metastatic Cancer: Using Individual Participant Data to Inform Future Trials Methodology. Journal of the National Cancer Institute, 114:819-828, 2022
45. Shitara K, Doi T, Hosaka H, Thuss-Patience P, Santoro A, Longo F, Ozyilkan O, Cicin I, Park D, Zaanan A, Pericay C, Özgüroğlu M, Alsina M, Makris L, Benhadji KA, Ilson DH. Efficacy and safety of trifluridine/tipiracil in older and younger patients with metastatic gastric or gastroesophageal junction cancer: subgroup analysis of a randomized phase 3 study (TAGS). Gastric cancer, 25:586-597, 2022
46. Du J, Kageyama SI, Yamashita R, Hirata H, Hakozaki Y, Okumura M, Motegi A, Hojo H, Nakamura M, Hirano Y, Sunakawa H, Minamide T, Kotani D, Tanaka K, Yano T, Kojima T, Ohashi A, Tsuchihara K, Akimoto T. Impacts of the STING-IFNAR1-STAT1-IRF1 pathway on the cellular immune reaction induced by fractionated irradiation. Cancer science, 113:1352-1361, 2022
47. Matsumura M, Hasegawa K, Oba M, Yamaguchi K, Uetake H, Yoshino T, Morita S, Takahashi K, Unno M, Shimada Y, Muro K, Matsuhashi N, Mori M, Baba H, Shimada M, Mise Y, Kawaguchi Y, Kagimura T, Ishigure K, Saiura A, Sugihara K, Kokudo N. A randomized controlled trial of surgery and postoperative modified FOLFOX6 versus surgery and perioperative modified FOLFOX6 plus cetuximab in patients with KRAS wild-type resectable colorectal liver metastases: EXPERT study. Langenbeck’s archives of surgery, 407:1345-1356, 2022
48. Strickler JH, Yoshino T, Graham RP, Siena S, Bekaii-Saab T. Diagnosis and Treatment of ERBB2-Positive Metastatic Colorectal Cancer: A Review. JAMA oncology, 8:760-769, 2022
49. Chida K, Kawazoe A, Suzuki T, Kawazu M, Ueno T, Takenouchi K, Nakamura Y, Kuboki Y, Kotani D, Kojima T, Bando H, Mishima S, Kuwata T, Sakamoto N, Watanabe J, Mano H, Ikeda M, Shitara K, Endo I, Nakatsura T, Yoshino T. Transcriptomic Profiling of MSI-H/dMMR Gastrointestinal Tumors to Identify Determinants of Responsiveness to Anti-PD-1 Therapy. Clinical cancer research, 28:2110-2117, 2022
50. Maron SB, Moya S, Morano F, Emmett MJ, Chou JF, Sabwa S, Walch H, Peterson B, Schrock AB, Zhang L, Janjigian YY, Chalasani S, Ku GY, Disel U, Enzinger P, Uboha N, Kato S, Yoshino T, Shitara K, Nakamura Y, Saeed A, Kasi PM, Chao J, Lee J, Capanu M, Wainberg Z, Petty R, Pietrantonio F, Klempner SJ, Catenacci DVT. Epidermal Growth Factor Receptor Inhibition in Epidermal Growth Factor Receptor-Amplified Gastroesophageal Cancer: Retrospective Global Experience. Journal of clinical oncology, 40:2458-2467, 2022
51. Fujii S, Kotani D, Hattori M, Nishihara M, Shikanai T, Hashimoto J, Hama Y, Nishino T, Suzuki M, Yoshidumi A, Ueno M, Komatsu Y, Masuishi T, Hara H, Esaki T, Nakamura Y, Bando H, Yamada T, Yoshino T. Rapid Screening Using Pathomorphologic Interpretation to Detect BRAFV600E Mutation and Microsatellite Instability in Colorectal Cancer. Clinical cancer research, 28:2623-2632, 2022
52. Marshall JL, Peshkin BN, Yoshino T, Vowinckel J, Danielsen HE, Melino G, Tsamardinos I, Haudenschild C, Kerr DJ, Sampaio C, Rha SY, FitzGerald KT, Holland EC, Gallagher D, Garcia-Foncillas J, Juhl H. The Essentials of Multiomics. The oncologist, 27:272-284, 2022
53. Diaz LA Jr, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, de la Fourchardiere C, Rivera F, Elez E, Le DT, Yoshino T, Zhong WY, Fogelman D, Marinello P, Andre T. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study. The Lancet. Oncology, 23:659-670, 2022
54. Swain SM, Nishino M, Lancaster LH, Li BT, Nicholson AG, Bartholmai BJ, Naidoo J, Schumacher-Wulf E, Shitara K, Tsurutani J, Conte P, Kato T, Andre F, Powell CA. Multidisciplinary clinical guidance on trastuzumab deruxtecan (T-DXd)-related interstitial lung disease/pneumonitis-Focus on proactive monitoring, diagnosis, and management. Cancer treatment reviews, 106:102378, 2022
55. Yoshino T, Oki E, Misumi T, Kotaka M, Manaka D, Eto T, Hasegawa J, Takagane A, Nakamura M, Kato T, Munemoto Y, Nakamura F, Bando H, Taniguchi H, Sakamoto Y, Shiozawa M, Nishi M, Horiuchi T, Yamagishi H, Sakamoto J, Mizushima T, Ohtsu A, Mori M. Final Analysis of 3 Versus 6 Months of Adjuvant Oxaliplatin and Fluoropyrimidine-Based Therapy in Patients With Stage III Colon Cancer: The Randomized Phase III ACHIEVE Trial. Journal of clinical oncology, 40:3419-3429, 2022
56. Bando H, Nakamura Y, Taniguchi H, Shiozawa M, Yasui H, Esaki T, Kagawa Y, Denda T, Satoh T, Yamazaki K, Sunakawa Y, Kato T, Goto M, Yuki S, Nishina T, Oki E, Shinozaki E, Matsuhashi N, Takahashi N, Tsuji A, Ohtsubo K, Wakabayashi M, Ikeno T, Hata M, Odegaard JI, Yoshino T. Effects of Metastatic Sites on Circulating Tumor DNA in Patients With Metastatic Colorectal Cancer. JCO precision oncology, 6:e2100535, 2022
57. Myer NM, Shitara K, Chung HC, Lordick F, Kelly RJ, Szabo Z, Cao ZA, Leong S, Ilson DH, Weichert W. Evolution of predictive and prognostic biomarkers in the treatment of advanced gastric cancer. Journal of cancer research and clinical oncology, 148:2023-2043, 2022
58. Shah MA, Shitara K, Lordick F, Bang YJ, Tebbutt NC, Metges JP, Muro K, Lee KW, Shen L, Tjulandin S, Hays JL, Starling N, Xu RH, Sturtz K, Fontaine M, Oh C, Brooks E, Xu B, Li W, Li CJ, Borodyansky L, Van Cutsem E. Randomized, double-blind, placebo-controlled phase 3 study of paclitaxel {plus minus} napabucasin in pretreated advanced gastric or gastroesophageal junction adenocarcinoma. Clinical cancer research, 28:3686-3694, 2022
59. Nakamura Y, Olsen S, Zhang N, Liao J, Yoshino T. Comprehensive Genomic Profiling of Circulating Tumor DNA in Patients with Previously Treated Metastatic Colorectal Cancer: Analysis of a Real-World Healthcare Claims Database. Current oncology (Toronto, Ont.), 29:3433-3448, 2022
60. Narita Y, Yoshimoto T, Namai T, Asakawa T, Kawakami S, Gower-Page C, Reyes-Rivera I, Patel A, Nakamura Y. Pertuzumab Plus Trastuzumab for Treatment-Refractory HER2-Amplified Metastatic Colorectal Cancer: Comparison of the MyPathway Trial With a Real-World External Control Arm. JCO clinical cancer informatics, 6:e2200022, 2022
61. Lee KW, Van Cutsem E, Bang YJ, Fuchs CS, Kudaba I, Garrido M, Chung HC, Lee J, Castro HR, Chao J, Wainberg ZA, Cao ZA, Aurora-Garg D, Kobie J, Cristescu R, Bhagia P, Shah S, Tabernero J, Shitara K, Wyrwicz L. Association of Tumor Mutational Burden with Efficacy of Pembrolizumab±Chemotherapy as First-Line Therapy for Gastric Cancer in the Phase III KEYNOTE-062 Study. Clinical cancer research, 28:3489-3498, 2022
62. Yukami H, Kawazoe A, Lin YT, Koyama S, Fukuoka S, Hara H, Takahashi N, Kojima T, Asayama M, Yoshii T, Bando H, Kotani D, Nakamura Y, Kuboki Y, Mishima S, Wakabayashi M, Kuwata T, Goto M, Higuchi K, Yoshino T, Doi T, Nishikawa H, Shitara K. Updated Efficacy Outcomes of Anti-PD-1 Antibodies plus Multikinase Inhibitors for Patients with Advanced Gastric Cancer with or without Liver Metastases in Clinical Trials. Clinical cancer research, 28:3480-3488, 2022
63. Yoshino T, Van Cutsem E, Li J, Shen L, Kim TW, Sriuranpong V, Xuereb L, Aubel P, Fougeray R, Cattan V, Amellal N, Ohtsu A, Mayer RJ. Effect of KRAS codon 12 or 13 mutations on survival with trifluridine/tipiracil in pretreated metastatic colorectal cancer: a meta-analysis. ESMO open, 7:100511, 2022
64. Iwasa S, Bando H, Piao Y, Yoshizawa K, Yamaguchi K. The clinical position of ramucirumab-containing regimens for advanced gastric cancer: a review of clinical trial data. Future oncology (London, England), 18:2709-2721, 2022
65. Aoki Y, Kawazoe A, Kubota Y, Chida K, Mishima S, Kotani D, Nakamura Y, Kuboki Y, Bando H, Kojima T, Doi T, Yoshino T, Kuwata T, Shitara K. Characteristics and clinical outcomes of patients with advanced gastric or gastroesophageal cancer treated in and out of randomized clinical trials of first-line immune checkpoint inhibitors. International journal of clinical oncology, 27:1413-1420, 2022
66. Funada S, Luo Y, Kataoka Y, Yoshioka T, Fujita Y, Yoshida S, Katsura M, Tada M, Nishioka N, Nakamura Y, Furukawa TA. Inadequate reporting of adjudicators in open-label trials of anticancer drugs between 2017 and 2021: a methodological review. Journal of clinical epidemiology, 150:80-89, 2022
67. Shah MA, Shitara K, Lordick F, Bang YJ, Tebbutt NC, Metges JP, Muro K, Lee KW, Shen L, Tjulandin S, Hays JL, Starling N, Xu RH, Sturtz K, Fontaine M, Oh C, Brooks EM, Xu B, Li W, Li CJ, Borodyansky L, Van Cutsem E. Randomized, Double-Blind, Placebo-Controlled Phase III Study of Paclitaxel ± Napabucasin in Pretreated Advanced Gastric or Gastroesophageal Junction Adenocarcinoma. Clinical cancer research, OF1-OF9, 2022
68. Milella M, Lawlor RT, Luchini C, Johns AL, Casolino R, Yoshino T, Biankin AV, Scarpa A. ICGC-ARGO precision medicine: an update on familial matters in pancreatic cancer. The Lancet. Oncology, 23:991-992, 2022
69. Ikeda M, Uetake H, Yoshino T, Hata T, Oba MS, Takita A, Kimura T. Incidence and risk factors for venous thromboembolism, bleeding, and death in colorectal cancer (Cancer-VTE Registry). Cancer science, 113:3901-3911, 2022
70. Dankner M, Wang Y, Fazelzad R, Johnson B, Nebhan CA, Dagogo-Jack I, Myall NJ, Richtig G, Bracht JWP, Gerlinger M, Shinozaki E, Yoshino T, Kotani D, Fangusaro JR, Gautschi O, Mazieres J, Sosman JA, Kopetz S, Subbiah V, Davies MA, Groover AL, Sullivan RJ, Flaherty KT, Johnson DB, Benedetti A, Cescon DW, Spreafico A, Zogopoulos G, Rose AAN. Clinical Activity of Mitogen-Activated Protein Kinase-Targeted Therapies in Patients With Non-V600 BRAF-Mutant Tumors. JCO precision oncology, 6:e2200107, 2022
71. Franko J, Yin J, Adams RA, Zalcberg J, Fiskum J, Van Cutsem E, Goldberg RM, Hurwitz H, Bokemeyer C, Kabbinavar F, Curtis A, Meyers J, Chibaudel B, Yoshino T, de Gramont A, Shi Q. Trajectories of body weight change and survival among patients with mCRC treated with systemic therapy: Pooled analysis from the ARCAD database. European journal of cancer (Oxford, England : 1990), 174:142-152, 2022
72. Tabernero J, Shen L, Elimova E, Ku G, Liu T, Shitara K, Lin X, Boyken L, Li H, Grim J, Ajani J. HERIZON-GEA-01: Zanidatamab + chemo ± tislelizumab for 1L treatment of HER2-positive gastroesophageal adenocarcinoma. Future oncology (London, England), 18:3255-3266, 2022
73. Yoon HH, Jin Z, Kour O, Kankeu Fonkoua LA, Shitara K, Gibson MK, Prokop LJ, Moehler M, Kang YK, Shi Q, Ajani JA. Association of PD-L1 Expression and Other Variables With Benefit From Immune Checkpoint Inhibition in Advanced Gastroesophageal Cancer: Systematic Review and Meta-analysis of 17 Phase 3 Randomized Clinical Trials. JAMA oncology, 8:1456-1465, 2022
74. Mlecnik B, Torigoe T, Bindea G, Popivanova B, Xu M, Fujita T, Hazama S, Suzuki N, Nagano H, Okuno K, Hirohashi Y, Furuhata T, Takemasa I, Patel P, Vora H, Shah B, Patel JB, Rajvik KN, Pandya SJ, Shukla SN, Wang Y, Zhang G, Yoshino T, Taniguchi H, Bifulco C, Lugli A, Lee JJ, Zlobec I, Rau TT, Berger MD, Nagtegaal ID, Vink-Börger E, Hartmann A, Geppert CI, Kolwelter J, Merkel S, Grützmann R, Van den Eynde M, Jouret-Mourin A, Kartheuser A, Léonard D, Remue C, Wang J, Bavi P, Roehrl MHA, Ohashi PS, Nguyen LT, Han S, MacGregor HL, Hafezi-Bakhtiari S, Wouters BG, Masucci GV, Andersson E, Zavadova E, Vocka M, Spacek J, Petruzelka L, Konopasek B, Dundr P, Skalova H, Nemejcova K, Botti G, Tatangelo F, Delrio P, Ciliberto G, Maio M, Laghi L, Grizzi F, Marliot F, Fredriksen T, Buttard B, Lafontaine L, Maby P, Majdi A, Hijazi A, El Sissy C, Kirilovsky A, Berger A, Lagorce C, Paustian C, Ballesteros-Merino C, Dijkstra J, Van de Water C, van Lent-van Vliet S, Knijn N, Muşină AM, Scripcariu DV, Marincola FM, Ascierto PA, Fox BA, Pagès F, Kawakami Y, Galon J. Clinical Performance of the Consensus Immunoscore in Colon Cancer in the Asian Population from the Multicenter International SITC Study. Cancers, 14:4346, 2022
75. Krebs MG, Malapelle U, André F, Paz-Ares L, Schuler M, Thomas DM, Vainer G, Yoshino T, Rolfo C. Practical Considerations for the Use of Circulating Tumor DNA in the Treatment of Patients With Cancer: A Narrative Review. JAMA oncology, 8:1830-1839, 2022
76. Kubota Y, Aoki Y, Kawazoe A, Shitara K. Role of Nivolumab in the Management of First-Line Unresectable Advanced or Recurrent Gastric Cancer in Combination with Chemotherapy: Lessons from the Japanese Experience. Cancer management and research, 14:3083-3094, 2022
77. El Helali A, Tao J, Wong CHL, Chan WW, Mok KC, Wu WF, Shitara K, Mohler M, Boku N, Pang H, Lam KO. A meta-analysis with systematic review: Efficacy and safety of immune checkpoint inhibitors in patients with advanced gastric cancer. Frontiers in oncology, 12:908026, 2022
78. Yoshikawa A, Nakamura Y. Molecular Basis of HER2-Targeted Therapy for HER2-Positive Colorectal Cancer. Cancers, 15:183, 2022
79. Morinaga T, Inozume T, Kawazu M, Ueda Y, Sax N, Yamashita K, Kawashima S, Nagasaki J, Ueno T, Lin J, Ohara Y, Kuwata T, Yukami H, Kawazoe A, Shitara K, Honobe-Tabuchi A, Ohnuma T, Kawamura T, Umeda Y, Kawahara Y, Nakamura Y, Kiniwa Y, Morita A, Ichihara E, Kiura K, Enokida T, Tahara M, Hasegawa Y, Mano H, Suzuki Y, Nishikawa H, Togashi Y. Mixed Response to Cancer Immunotherapy is Driven by Intratumor Heterogeneity and Differential Interlesion Immune Infiltration. Cancer research communications, 2:739-753, 2022
