Annual Report 2022
Department of Hepatobiliary and Pancreatic Oncology
Masafumi Ikeda, Shuichi Mitsunaga, Hiroshi Imaoka, Mitsuhito Sasaki, Kazuo Watanabe, Tomoyuki Satake, Taro Shibuki, Kei Okumura, Hiroki Eguchi, Kanae Inoue, Tomonao Taira, Shota Yamaguchi
Introduction
The Department of Hepatobiliary and Pancreatic Oncology is responsible for the diagnosis and treatment of patients with hepatic, biliary, and pancreatic cancers as well as interventional management by endoscopic or percutaneous procedures (Table 1). Our goal is to provide high-quality cancer treatment with adequate palliative care, and to develop novel and effective treatments and procedures through well-designed clinical trials and research.
The Team and What We Do
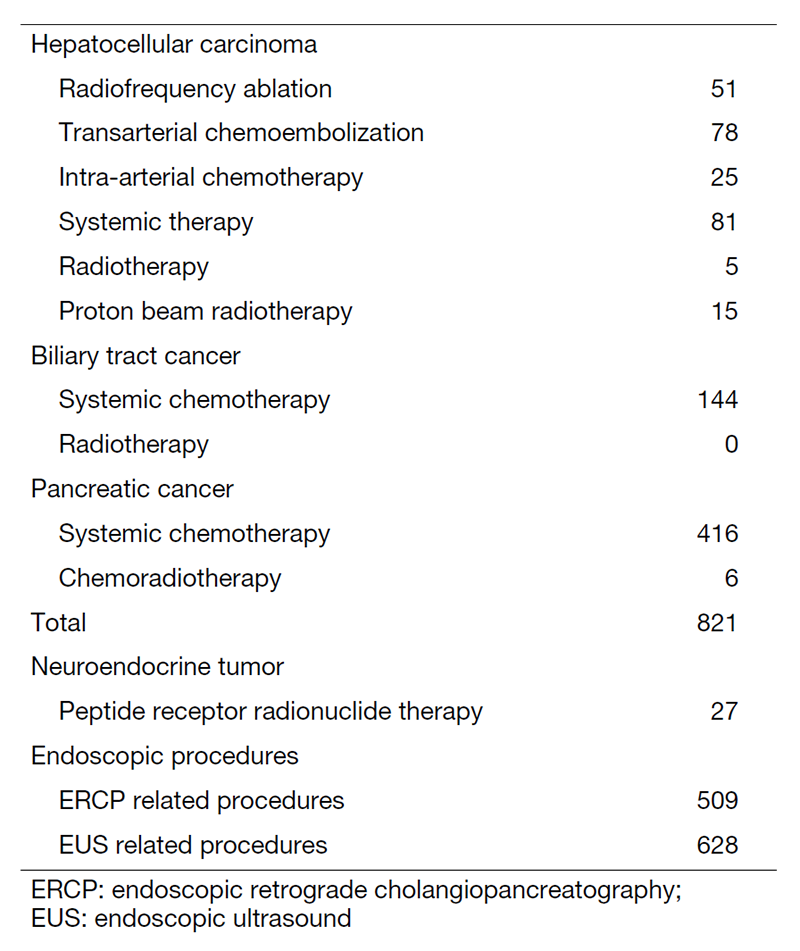
Our department consists of 6 staff oncologists and 6 residents, with an average of 38 beds in the hospital. These doctors are grouped into two teams and each team determines daily treatment plans for each admitted patient, and all doctors discuss with overall treatment strategy for all admitted patients in our department once a week. The major treatment strategies for individual patients are discussed in weekly tumor board conferences attended by medical oncologists, surgeons, radiologists, radiation oncologists, and pharmacists. We are also responsible for peptide receptor radionuclide therapy for neuroendocrine tumors, endoscopic abdominal ultrasonographic examinations, endoscopic or percutaneous ultrasound-guided biopsies of abdominal masses, local ablative therapy for liver tumors, endoscopic or percutaneous biliary and abscess drainage, and obstructive jaundice stenting, etc (Table 2).
Table 1. Number of cancer patients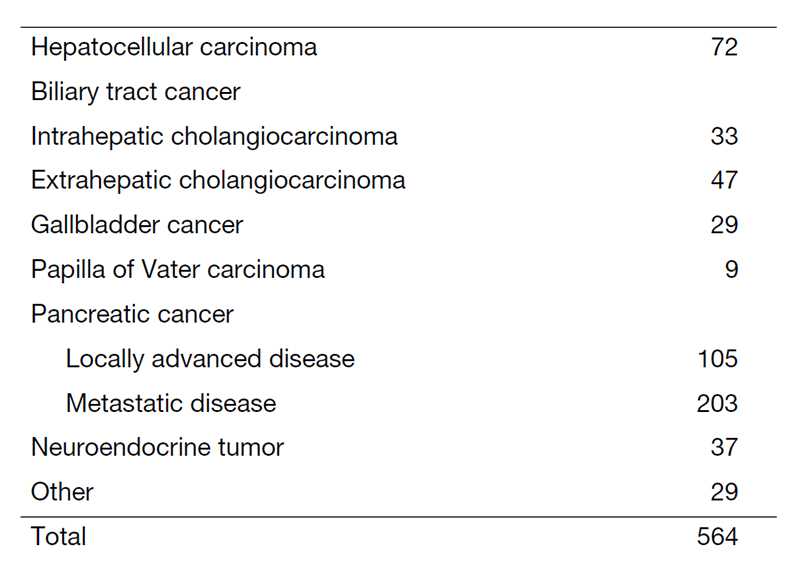

Research Activities
Hepatocellular carcinoma (HCC)
Systemic therapy is one of the most important treatment modalities for advanced hepatocellular carcinoma (HCC). At present, durvalumab plus tremelimumab and durvalumab alone have been approved and are now available in addition to six regimens, including atezolizumab plus bevacizumab, sorafenib, and lenvatinib as a first-line treatment, regorafenib, cabozantinib, and ramucirumab as a second or later line treatment. Treatment selection for these regimens and the mechanism related to treatment resistance are important issues to be addressed during the patient treatment journey. We initiated the biomarker study of angiogenetic and immunogenic factors for unresectable HCC (PRISM-Bio) to clarify them.
Biliary tract cancer (BTC)
Adjuvant therapy remains unestablished for resected BTC. A phase III trial of S-1 vs. observation was conducted as a JCOG1202 trial. Adjuvant S-1 demonstrated a significantly longer survival than observation and became the standard of care in Japan. For unresectable BTC, current development is mainly focused on immunotherapy. Occupational cholangiocarcinoma is an immune hot tumor that develops from long-term exposure to 1,2-dichloropropane and/or dichloromethane. We reported that 3 out of 3 patients achieved a complete response after treatment with nivolumab, including the results of immunopathological and genetic analyses.
Pancreatic cancer (PC)
For unresectable PC, gemcitabine plus nab-paclitaxel and FOLFIRINOX for first-line chemotherapy, nano-liposomal irinotecan plus 5-fluorouracil/leucovorin for second-line chemotherapy have been approved in Japan. We conducted a phase I/II trial of nano-liposomal irinotecan plus S-1, using oral S-1 instead of 5-fluorouracil/leucovorin, in second-line setting. The enrollment has already been completed and we are awaiting the final analysis.
Others
We launched a master protocol study with multiple cohorts to establish treatments for rare fractions of hepatobiliary and pancreatic cancers and neuroendocrine tumors. Initially, three cohorts (Cohort A: gemcitabine plus nab-paclitaxel for pancreatic anaplastic cancer; Cohort B: mFOLFIRINOX for pancreatic acinar cell carcinoma; Cohort C: FOLFIRINOX for pancreatic cancer with UGT1A1 double variant) were opened.
Clinical Trials
Eighty-five clinical trials (sponsored: 40 trials, investigator-initiated: 45 trials) are ongoing, and 10 clinical trials (sponsored: 3 trials, investigator-initiated: 7 trials) are being planned for the upcoming year.
HCC
The developments of new regimen are actively ongoing by triplet and quatro regimens as well as doublet regimens, such as combination therapy of VEGFR-targeted multi-kinase inhibitors or antibodies, and immune checkpoint inhibitors. Some clinical trials of new agents of anti-Glypican3/CD3 bispecific antibody (ERY974) plus atezolizumab plus bevacizumab and lenvatinib plus coformulated pembrolizumab/quavonlimab (MK-1308A) as the first-line therapy, lenvatinib plus beta-catenin modulator (E7386) and anti-DLK-1 antibody (CBA-1205) as the second- or later-line therapy, and durvalumab plus tremelimumab plus lenvatinib as combined therapy with transarterial chemoembolization, are underway. A prospective observational study, PRISM, was conducted to collect real-world data in Japan and establish real-world evidence.
BTC
As first-line systemic therapy, gemcitabine (Gem) plus cisplatin (GC) plus durvalumab became the first-line regimen in addition to GC, Gem plus S-1 therapy (GS), and Gem plus cisplatin plus S-1 therapy (GCS). Additionally, precision medicine based on genomic alterations is also focused on BTC. The enrollments of some sponsored trials of JPH203 for anticancer drugs that target L-type amino acid transporter 1, E7090 for FGFR2 fusion, and LY3410738 for IDH1 mutation for second- or later-line advanced BTCs were terminated. Among them, JPH203, navuranlat, demonstrated favorable efficacy in patients with heavily pre-treated BTC, and the efficacy was enhanced in patients with high LAT1 expression.
PC
Some investigators-initiated trials of a phase II/III trial of neoadjuvant S-1 and concurrent radiotherapy vs. Gem plus nab-paclitaxel for borderline resectable PC (GABARNANCE), a phase III trial of concurrent S-1 and radiotherapy combined with nivolumab vs. S-1 and radiotherapy alone for locally advanced or borderline resectable PC (JCOG1908E), Gem plus nab-paclitaxel vs. modified FOLFIRINOX vs. S-IROX for metastatic PC (JCOG1611), as the first line setting were ongoing. Some sponsored trials of FOLFIRINOX or Gem plus nab-paclitaxel with molecular targeted agents or immune checkpoint inhibitors were under recruitment. Precision medicine for NRG1 fusion and KRASG12C mutation are ongoing as sponsored clinical trials.
Neuroendocrine tumor
A phase III trial of everolimus plus lanreotide vs. everolimus (investigator-initiated trial) and a phase II trial of surufatinib (sponsored trial) are ongoing for gastroenterol pancreatic neuroendocrine tumors.
Others
Some investigator-initiated studies using circulating tumor DNA are ongoing for hepatobiliary & pancreatic cancer.
Education
Our trainees are provided with daily training with group discussions on the daily practice of inpatient and outpatient management. They can learn the indications, administration and the management of adverse events from loco-regional treatments to systemic therapy for patients with hepatic, biliary, and pancreatic cancer and the accompanying procedures to undertake diagnosis and interventional management, and provide outpatient care. Trainees conduct some retrospective clinical researches and prospective clinical trials to resolve some clinical questions arising from daily practice. Further, they can make presentations of their research in domestic and overseas meetings and publish papers in English under the instruction of staff physicians.
Future Prospects
Immunotherapy has become the standard treatment for HCC and BTC. The number of patients with HCC in Japan is on the decline and the number of patients with BTC has plateaued. However, the numbers of the annual incidence of these cancers remain higher as compared to the other countries. Cytotoxic agents in PC remain the main treatment, and the efficacy of standard treatments is limited. However, the number of patients with PC is rapidly increasing. Developing new agents is an urgent need in the field of hepatobiliary and pancreatic cancer, and Japanese oncologists are expected to play a crucial role in new agent development. Therefore, we actively participate in drug development from the early phases, lead domestic clinical trials, and plan international clinical trials collaborating with overseas investigators. In addition, it is necessary to develop biomarker research and endoscopic management alongside cancer treatment.
List of papers published in 2022
Journal
1. Ozaka M, Nakachi K, Kobayashi S, Ohba A, Imaoka H, Terashima T, Ishii H, Mizusawa J, Katayama H, Kataoka T, Okusaka T, Ikeda M, Sasahira N, Miwa H, Mizukoshi E, Okano N, Mizuno N, Yamamoto T, Komatsu Y, Todaka A, Kamata K, Furukawa M, Fujimori N, Katanuma A, Takayama Y, Tsumura H, Fukuda H, Ueno M, Furuse J. A randomised phase II study of modified FOLFIRINOX versus gemcitabine plus nab-paclitaxel for locally advanced pancreatic cancer (JCOG1407). European journal of cancer (Oxford, England : 1990), 181:135-144, 2023
2. Matsuzaki J, Kato K, Oono K, Tsuchiya N, Sudo K, Shimomura A, Tamura K, Shiino S, Kinoshita T, Daiko H, Wada T, Katai H, Ochiai H, Kanemitsu Y, Takamaru H, Abe S, Saito Y, Boku N, Kondo S, Ueno H, Okusaka T, Shimada K, Ohe Y, Asakura K, Yoshida Y, Watanabe SI, Asano N, Kawai A, Ohno M, Narita Y, Ishikawa M, Kato T, Fujimoto H, Niida S, Sakamoto H, Takizawa S, Akiba T, Okanohara D, Shiraishi K, Kohno T, Takeshita F, Nakagama H, Ota N, Ochiya T. Prediction of tissue-of-origin of early stage cancers using serum miRNomes. JNCI cancer spectrum, 7:pkac080, 2023
3. Furuse J, Ueno M, Ikeda M, Okusaka T, Teng Z, Furuya M, Ioka T. Liposomal irinotecan with fluorouracil and leucovorin after gemcitabine-based therapy in Japanese patients with metastatic pancreatic cancer: additional safety analysis of a randomized phase 2 trial. Japanese journal of clinical oncology, 53:130-137, 2023
4. Nakachi K, Ikeda M, Konishi M, Nomura S, Katayama H, Kataoka T, Todaka A, Yanagimoto H, Morinaga S, Kobayashi S, Shimada K, Takahashi Y, Nakagohri T, Gotoh K, Kamata K, Shimizu Y, Ueno M, Ishii H, Okusaka T, Furuse J. Adjuvant S-1 compared with observation in resected biliary tract cancer (JCOG1202, ASCOT): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet (London, England), 401:195-203, 2023
5. Umemoto K, Sunakawa Y, Ueno M, Furukawa M, Mizuno N, Sudo K, Kawamoto Y, Kajiwara T, Ohtsubo K, Okano N, Matsuhashi N, Itoh S, Matsumoto T, Shimizu S, Otsuru T, Hasegawa H, Okuyama H, Ohama H, Moriwaki T, Ohta T, Odegaard JI, Nakamura Y, Bando H, Yoshino T, Ikeda M, Morizane C. Clinical significance of circulating-tumour DNA analysis by metastatic sites in pancreatic cancer. British journal of cancer, 128:1603-1608, 2023
6. Okusaka T, Nakamura M, Yoshida M, Kitano M, Ito Y, Mizuno N, Hanada K, Ozaka M, Morizane C, Takeyama Y. Clinical Practice Guidelines for Pancreatic Cancer 2022 from the Japan Pancreas Society: a synopsis. International journal of clinical oncology, 28:493-511, 2023
7. Nakazawa J, Tsuruta N, Shimokawa M, Kawahira M, Arima S, Ido A, Koga F, Ueda Y, Komori A, Otsu S, Fukahori M, Makiyama A, Taguchi H, Honda T, Shibuki T, Nio K, Ide Y, Ureshino N, Mizuta T, Otsuka T, Shirakawa T, Mitsugi K. Multicenter Retrospective Analysis of Original versus Modified FOLFIRINOX in Metastatic Pancreatic Cancer: Results of the NAPOLEON Study. Oncology, 101:22-31, 2023
8. Takahara N, Nakai Y, Isayama H, Sasaki T, Morine Y, Watanabe K, Ueno M, Ioka T, Kanai M, Kondo S, Okano N, Koike K. A prospective multicenter phase II study of FOLFIRINOX as a first-line treatment for patients with advanced and recurrent biliary tract cancer. Investigational new drugs, 41:76-85, 2023
9. Sekine M, Hashimoto Y, Shibuki T, Okumura K, Kobori I, Miyagaki A, Sasaki Y, Takano Y, Matsumoto K, Mashima H. A retrospective multicenter study comparing the punctures to B2 and B3 in endoscopic ultrasound-guided hepaticogastrostomy. DEN open, 3:e201, 2023
10. Hsu C, Ducreux M, Zhu AX, Qin S, Ikeda M, Kim TY, Galle PR, Finn RS, Chen E, Ma N, Hu Y, Li L, Cheng AL. Hepatic Events and Viral Kinetics in Hepatocellular Carcinoma Patients Treated with Atezolizumab plus Bevacizumab. Liver cancer, 12:44-56, 2023
11. Ide Y, Otsuka T, Shimokawa M, Koga F, Ueda Y, Nakazawa J, Komori A, Otsu S, Arima S, Fukahori M, Makiyama A, Shinohara Y, Ueno S, Taguchi H, Honda T, Shibuki T, Nio K, Ureshino N, Mizuta T, Mitsugi K, Shirakawa T. Conversion Surgery for Unresectable Pancreatic Cancer Treated With FOLFIRINOX or Gemcitabine Plus Nab-paclitaxel. Anticancer research, 43:1817-1826, 2023
12. Shibuki T, Okumura K, Sekine M, Kobori I, Miyagaki A, Sasaki Y, Takano Y, Hashimoto Y. Covered self-expandable metallic stents versus plastic stents for endoscopic ultrasound-guided hepaticogastrostomy in patients with malignant biliary obstruction. Clinical endoscopy, 2023
13. Ohba A, Morizane C, Ueno M, Kobayashi S, Kawamoto Y, Komatsu Y, Ikeda M, Sasaki M, Okano N, Furuse J, Hiraoka N, Yoshida H, Kuchiba A, Sadachi R, Nakamura K, Matsui N, Nakamura Y, Okamoto W, Yoshino T, Okusaka T. Multicenter phase II trial of trastuzumab deruxtecan for HER2-positive unresectable or recurrent biliary tract cancer: HERB trial. Future oncology (London, England), 18:2351-2360, 2022
14. Sato K, Fujita T, Matsuzaki H, Takeshita N, Fujiwara H, Mitsunaga S, Kojima T, Mori K, Daiko H. Real-time detection of the recurrent laryngeal nerve in thoracoscopic esophagectomy using artificial intelligence. Surgical endoscopy, 36:5531-5539, 2022
15. Morizane C, Machida N, Honma Y, Okusaka T, Boku N, Kato K, Nomura S, Hiraoka N, Sekine S, Taniguchi H, Okano N, Yamaguchi K, Sato T, Ikeda M, Mizuno N, Ozaka M, Kataoka T, Ueno M, Kitagawa Y, Terashima M, Furuse J. Effectiveness of Etoposide and Cisplatin vs Irinotecan and Cisplatin Therapy for Patients With Advanced Neuroendocrine Carcinoma of the Digestive System: The TOPIC-NEC Phase 3 Randomized Clinical Trial. JAMA oncology, 8:1447-1455, 2022
16. Okusaka T, Kudo M, Ikeda K, Ikeda M, Okita K, Sugawara M, Tamai T, Ren M, Saito K, Kumada H. Impact of bodyweight-based starting doses on the safety and efficacy of lenvatinib in primarily Japanese patients with hepatocellular carcinoma. Hepatology research, 52:784-793, 2022
17. Ohba A, Ueno H, Shiba S, Okano N, Kobayashi T, Nagashima F, Sasahira N, Sasaki M, Imaoka H, Sakamoto Y, Kondo S, Morizane C, Ozaka M, Ikeda M, Furuse J, Okusaka T. Safety and efficacy of S-IROX (S-1, irinotecan and oxaliplatin combination therapy) in patients with advanced pancreatic cancer: A multicenter phase 1b dose-escalation and dose-expansion clinical trial. European journal of cancer (Oxford, England : 1990), 174:40-47, 2022
18. Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, Tsumura H, Kuzuya T, Isoda N, Moriguchi M, Aino H, Ido A, Kawabe N, Nakao K, Wada Y, Ogasawara S, Yoshimura K, Okusaka T, Furuse J, Kokudo N, Okita K, Johnson PJ, Arai Y. Final Results of TACTICS: A Randomized, Prospective Trial Comparing Transarterial Chemoembolization Plus Sorafenib to Transarterial Chemoembolization Alone in Patients with Unresectable Hepatocellular Carcinoma. Liver cancer, 11:354-367, 2022
19. Terashima T, Morizane C, Ushiama M, Shiba S, Takahashi H, Ikeda M, Mizuno N, Tsuji K, Yasui K, Azemoto N, Satake H, Nomura S, Yachida S, Sugano K, Furuse J. Germline variants in cancer-predisposing genes in pancreatic cancer patients with a family history of cancer. Japanese journal of clinical oncology, 52:1105-1114, 2022
20. Yoshida Y, Kobayashi S, Ueno M, Morizane C, Tsuji K, Maruki Y, Mori K, Watanabe K, Ohba A, Furuta M, Todaka A, Tsujimoto A, Ozaka M, Okano N, Yane K, Umemoto K, Kawamoto Y, Terashima T, Tsumura H, Doi K, Shioji K, Asagi A, Kojima Y, Suzuki E, Toshiyama R, Furukawa M, Naganuma A, Suzuki R, Miwa H, Ikeda M, Furuse J. Efficacy of chemotherapy for patients with metastatic or recurrent pancreatic adenosquamous carcinoma: A multicenter retrospective analysis. Pancreatology, 22:1159-1166, 2022
21. Satake T, Morizane C, Rikitake R, Higashi T, Okusaka T, Kawai A. The epidemiology of rare types of hepatobiliary and pancreatic cancer from national cancer registry. Journal of gastroenterology, 57:890-901, 2022
22. Ikeda M, Arai Y, Inaba Y, Tanaka T, Sugawara S, Kodama Y, Aramaki T, Anai H, Morita S, Tsukahara Y, Seki H, Sato M, Kamimura K, Azama K, Tsurusaki M, Sugihara E, Miyazaki M, Kobayashi T, Sone M. Conventional or Drug-Eluting Beads? Randomized Controlled Study of Chemoembolization for Hepatocellular Carcinoma: JIVROSG-1302. Liver cancer, 11:440-450, 2022
23. Miura T, Mitsunaga S, Matsuzaki J, Takizawa S, Kato K, Ochiai A, Ochiya T. Serum microRNAs as new criteria for referral to early palliative care services in treatment-naïve advanced cancer patients. Oncotarget, 13:1341-1349, 2022
24. Sato K, Fujita T, Matsuzaki H, Takeshita N, Fujiwara H, Mitsunaga S, Kojima T, Mori K, Daiko H. Correction to: Real-time detection of the recurrent laryngeal nerve in thoracoscopic esophagectomy using artificial intelligence. Surgical endoscopy, 36:9483, 2022
25. Sasaki K, Ito M, Kobayashi S, Kitaguchi D, Matsuzaki H, Kudo M, Hasegawa H, Takeshita N, Sugimoto M, Mitsunaga S, Gotohda N. Automated surgical workflow identification by artificial intelligence in laparoscopic hepatectomy: Experimental research. International journal of surgery (London, England), 105:106856, 2022
26. Chida K, Kawazoe A, Suzuki T, Kawazu M, Ueno T, Takenouchi K, Nakamura Y, Kuboki Y, Kotani D, Kojima T, Bando H, Mishima S, Kuwata T, Sakamoto N, Watanabe J, Mano H, Ikeda M, Shitara K, Endo I, Nakatsura T, Yoshino T. Transcriptomic Profiling of MSI-H/dMMR Gastrointestinal Tumors to Identify Determinants of Responsiveness to Anti-PD-1 Therapy. Clinical cancer research, 28:2110-2117, 2022
27. Takahashi S, Ohno I, Ikeda M, Konishi M, Kobayashi T, Akimoto T, Kojima M, Morinaga S, Toyama H, Shimizu Y, Miyamoto A, Tomikawa M, Takakura N, Takayama W, Hirano S, Otsubo T, Nagino M, Kimura W, Sugimachi K, Uesaka K. Neoadjuvant S-1 With Concurrent Radiotherapy Followed by Surgery for Borderline Resectable Pancreatic Cancer: A Phase II Open-label Multicenter Prospective Trial (JASPAC05). Annals of surgery, 276:e510-e517, 2022
28. Koga F, Kawaguchi Y, Shimokawa M, Murayama K, Nakashita S, Oza N, Ureshino N, Takahashi H, Ueda Y, Nakazawa J, Komori A, Otsu S, Arima S, Fukahori M, Makiyama A, Taguchi H, Honda T, Shibuki T, Nio K, Ide Y, Mizuta T, Shirakawa T, Otsuka T, Mitsugi K. Gemcitabine plus nab-paclitaxel in older patients with metastatic pancreatic cancer: A post-hoc analysis of the real-world data of a multicenter study (the NAPOLEON study). Journal of geriatric oncology, 13:82-87, 2022
29. Yachida S, Totoki Y, Noë M, Nakatani Y, Horie M, Kawasaki K, Nakamura H, Saito-Adachi M, Suzuki M, Takai E, Hama N, Higuchi R, Hirono S, Shiba S, Kato M, Furukawa E, Arai Y, Rokutan H, Hashimoto T, Mitsunaga S, Kanda M, Tanaka H, Takata S, Shimomura A, Oshima M, Hackeng WM, Okumura T, Okano K, Yamamoto M, Yamaue H, Morizane C, Arihiro K, Furukawa T, Sato T, Kiyono T, Brosens LAA, Wood LD, Hruban RH, Shibata T. Comprehensive Genomic Profiling of Neuroendocrine Carcinomas of the Gastrointestinal System. Cancer discovery, 12:692-711, 2022
30. Shibuki T, Mizuta T, Shimokawa M, Koga F, Ueda Y, Nakazawa J, Komori A, Otsu S, Arima S, Fukahori M, Makiyama A, Taguchi H, Honda T, Mitsugi K, Nio K, Ide Y, Ureshino N, Shirakawa T, Otsuka T. Prognostic nomogram for patients with unresectable pancreatic cancer treated with gemcitabine plus nab-paclitaxel or FOLFIRINOX: A post-hoc analysis of a multicenter retrospective study in Japan (NAPOLEON study). BMC cancer, 22:19, 2022
31. Nakajima H, Harano K, Nakai T, Kusuhara S, Nakao T, Funasaka C, Kondoh C, Matsubara N, Naito Y, Hosono A, Mitsunaga S, Ishii G, Mukohara T. Impacts of clinicopathological factors on efficacy of trastuzumab deruxtecan in patients with HER2-positive metastatic breast cancer. Breast (Edinburgh, Scotland), 61:136-144, 2022
32. Kudo M, Ikeda M, Ueshima K, Sakamoto M, Shiina S, Tateishi R, Nouso K, Hasegawa K, Furuse J, Miyayama S, Murakami T, Yamashita T, Kokudo N. Response Evaluation Criteria in Cancer of the liver version 6 (Response Evaluation Criteria in Cancer of the Liver 2021 revised version). Hepatology research, 52:329-336, 2022
33. Llovet JM, Singal AG, Villanueva A, Finn RS, Kudo M, Galle PR, Ikeda M, Callies S, McGrath LM, Wang C, Abada P, Widau RC, Gonzalez-Gugel E, Zhu AX. Prognostic and Predictive Factors in Patients with Advanced HCC and Elevated Alpha-Fetoprotein Treated with Ramucirumab in Two Randomized Phase III Trials. Clinical cancer research, 28:2297-2305, 2022
34. Suzuki H, Mitsunaga S, Ikeda M, Aoyama T, Yoshizawa K, Yamaguchi M, Suzuki M, Narita M, Kawasaki T, Ochiai A. Interleukin 6/gp130 axis promotes neural invasion in pancreatic cancer. Cancer medicine, 11:5001-5012, 2022
35. Tanaka S, Umemoto K, Kubo S, Sato Y, Mimaki S, Tsuchihara K, Takemura S, Shinkawa H, Mori A, Ikeda M. Nivolumab for treating patients with occupational cholangiocarcinoma. Journal of hepato-biliary-pancreatic sciences, 29:1153-1155, 2022
36. Goda K, Hashimoto Y, Ikeda M. Cholangioscopic diagnosis of hemobilia: an unusual case of left hepatic portal hypertension by plasma cell tumor. VideoGIE, 7:143-145, 2022
37. Kobayashi S, Suzuki M, Ueno M, Maruki Y, Okano N, Todaka A, Ozaka M, Tsuji K, Shioji K, Doi K, Kojima Y, Tsumura H, Tanaka K, Higuchi H, Kawabe K, Imaoka H, Yamashita T, Miwa H, Nagano H, Arima S, Hayashi H, Naganuma A, Yamaguchi H, Hisano T, Umemoto K, Ishii S, Nakashima K, Suzuki R, Kitano Y, Misumi T, Furuse J, Ishii H. Comparing the Efficacy and Safety of Gemcitabine plus Nab-Paclitaxel versus Gemcitabine Alone in Older Adults with Unresectable Pancreatic Cancer. The oncologist, 27:e774-e782, 2022
38. Kobori I, Hashimoto Y, Shibuki T, Okumura K, Sekine M, Miyagaki A, Sasaki Y, Takano Y, Katayama Y, Kuwada M, Gyotoku Y, Kusano Y, Tamano M. Safe Performance of Track Dilation and Bile Aspiration with ERCP Catheter in EUS-Guided Hepaticogastrostomy with Plastic Stents: A Retrospective Multicenter Study. Journal of clinical medicine, 11:4986, 2022
39. Irisawa A, Takeno M, Watanabe K, Takahashi H, Mitsunaga S, Ikeda M. Incidence of and risk factors for severe neutropenia during treatment with the modified FOLFIRINOX therapy in patients with advanced pancreatic cancer. Scientific reports, 12:15574, 2022
40. Itoh S, Ikeda M. Atezolizumab plus bevacizumab for patients with Child-Pugh-B in hepatocellular carcinoma. Hepatobiliary surgery and nutrition, 11:876-878, 2022
41. Otsuka T, Shirakawa T, Shimokawa M, Koga F, Kawaguchi Y, Ueda Y, Nakazawa J, Komori A, Otsu S, Arima S, Fukahori M, Okabe Y, Makiyama A, Taguchi H, Honda T, Shibuki T, Nio K, Ide Y, Mizuta T, Mitsugi K, Ureshino N. A multicenter propensity score analysis of FOLFIRINOX vs gemcitabine plus nab-paclitaxel administered to patients with metastatic pancreatic cancer: results from the NAPOLEON study. International journal of clinical oncology, 26:941-950, 2021
42. Ishii T, Suzuki A, Kuwata T, Hisamitsu S, Hashimoto H, Ohara Y, Yanagihara K, Mitsunaga S, Yoshino T, Kinoshita T, Ochiai A, Shitara K, Ishii G. Drug-exposed cancer-associated fibroblasts facilitate gastric cancer cell progression following chemotherapy. Gastric cancer, 24:810-822, 2021
43. Taguchi H, Otsuka T, Shimokawa M, Arima S, Hashimoto S, Ido A, Koga F, Ueda Y, Nakazawa J, Komori A, Otsu S, Fukahori M, Makiyama A, Honda T, Shibuki T, Mizuta T, Mitsugi K, Nio K, Ide Y, Ureshino N, Shirakawa T. Gemcitabine Plus Nanoparticle Albumin-bound Paclitaxel Versus FOLFIRINOX for Recurrent Pancreatic Cancer After Resection. Anticancer research, 41:3573-3582, 2021
