Annual Report 2022
Division of Cancer Immunology
Hiroyoshi Nishikawa, Yuka Maeda, Kosuke Tanaka, Keisuke Watanabe, Hitomi Nishinakamura, Kota Itahashi, Priya Saju, Megumi Fukuoka, Takeo Naito, Akihito Nagata, Nanae Shimoyama, Yuki Ishige, Kyo Ishin, May Thinzar Hlaing, Abdullah AimiNaim
Introduction
Cancer immunotherapies using immune checkpoint inhibitors (ICIs) or chimeric antigen receptor T (CAR-T) cells or T-cell receptor T (TCR-T) cells have become an established therapy for cancers in some patient population. However, not a few patients experience relapse or resistant disease. Cancers often create a complex immune suppressive environment that can impair the host-immune reaction, thereby impairing the efficacy of ICIs or adoptive transferred anti-tumor T cells. Therefore, there is an urgent demand for elucidating the mechanisms of resistance and identifying reliable biomarkers to predict its efficacy to make immunotherapies much more effective in clinical settings.
The Team and What We Do
We have been intensively collecting clinical samples from cancer patients treated with immunotherapies and investigating the mechanisms of cancer immunoreactions using multi-omics analysis, including flow cytometry, mass cytometry, imaging mass cytometry, single-cell transcriptome analysis, proteomics, and metabolomics (Figure 1). We are also developing first-in-class gene-modified, cell-based immunotherapies using CAR-T cell or TCR-T cell platforms targeting the above mechanisms of resistance (Figure 2).
Figure 1. Integrated multi-omics analyses on patient-derived tumor/immune cell samples.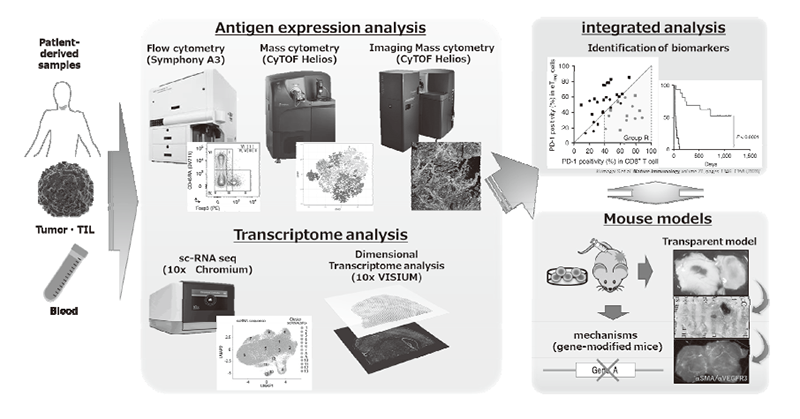

Figure 2. Drug discovery using chimeric antigen receptor T cell (CAR-T cell) format.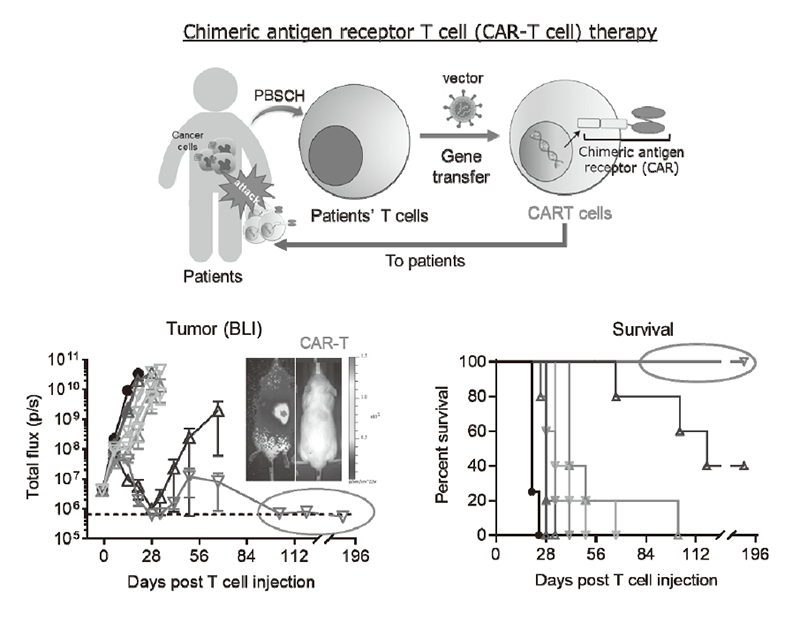

Research Activities
We have identified multiple mechanisms that impair the efficacy of cancer immunotherapies using patient-derived pre- and post-ICI treatment samples. In addition, we have elucidated differentiation process and epigenetic profiles of regulatory T cells (Treg cells) in the tumor microenvironment (TME). BATF was identified as a key regulator, which leveraged Treg cell differentiation through epigenetically controlling activation-associated gene expression, resulting in the robustness of Treg cells in the TME. We are also developing novel CAR-T cells that overcome multiple immunosuppressive factors to improve the treatment outcome of adoptive cell therapies in solid tumors. Some of them are under pre-clinical testing aiming for first-in-human trials. In addition, we have developed a combination therapy with a drug and anti-PD-1 Ab. This drug activates the innate immunity via Toll-like receptor signaling. For solid tumors that do not respond to anti-PD-1 antibody therapy, we analyzed the anti-tumor immune response by stimulating the innate immune response in tumor-bearing mice model. The phenotype and proportion of the various immune cells that infiltrate tumors have been identified, leading to the development of a novel combination cancer immunotherapy.
Clinical Trials
An investigator-initiated first-in-human trial of our CAR-T cell therapy is under preparation.
Education
Our division hosts graduate students from several universities with partnership agreements, and young residents from the National Cancer Center Hospital for training in the translational research. We strongly support their career development, and many alumni are leading the immunology field in a variety of posts in the clinic, academia, or paratheatrical companies.
Future Prospects
We will further investigate the mechanisms involved in tumor immune suppression or tumor immune evasion in various cancers with a broad view into such areas as immunology and genetics, using new approaches including special and dynamic analysis of the transcriptome or immune cell phenotypes, and transparent mouse tissue models. Our findings will lead to new drug discoveries to overcome resistance and to identify biomarkers in immune therapies. We are planning a first-in-human trial of CAR-T cell therapy developed in our lab.
List of papers published in 2022
Journal
1. Hiramatsu H, Nosaka K, Kusumoto S, Nakano N, Choi I, Yoshimitsu M, Imaizumi Y, Hidaka M, Sasaki H, Makiyama J, Ohtsuka E, Jo T, Ogata M, Ito A, Yonekura K, Tatetsu H, Kato T, Kawakita T, Suehiro Y, Ishitsuka K, Iida S, Matsutani T, Nishikawa H, Utsunomiya A, Ueda R, Ishida T. Landscape of immunoglobulin heavy chain γ gene class switch recombination in patients with adult T-cell leukemia-lymphoma. Haematologica, 108:1173-1178, 2023
2. Sugiyama D, Hinohara K, Nishikawa H. Significance of regulatory T cells in cancer immunology and immunotherapy. Experimental dermatology, 32:256-263, 2023
3. Kato S, Maeda Y, Sugiyama D, Watanabe K, Nishikawa H, Hinohara K. The cancer epigenome: Non-cell autonomous player in tumor immunity. Cancer science, 114:730-740, 2023
4. Itahashi K, Irie T, Yuda J, Kumagai S, Tanegashima T, Lin YT, Watanabe S, Goto Y, Suzuki J, Aokage K, Tsuboi M, Minami Y, Ishii G, Ohe Y, Ise W, Kurosaki T, Suzuki Y, Koyama S, Nishikawa H. BATF epigenetically and transcriptionally controls the activation program of regulatory T cells in human tumors. Science immunology, 7:eabk0957, 2022
5. Yukami H, Kawazoe A, Lin YT, Koyama S, Fukuoka S, Hara H, Takahashi N, Kojima T, Asayama M, Yoshii T, Bando H, Kotani D, Nakamura Y, Kuboki Y, Mishima S, Wakabayashi M, Kuwata T, Goto M, Higuchi K, Yoshino T, Doi T, Nishikawa H, Shitara K. Updated Efficacy Outcomes of Anti-PD-1 Antibodies plus Multikinase Inhibitors for Patients with Advanced Gastric Cancer with or without Liver Metastases in Clinical Trials. Clinical cancer research, 28:3480-3488, 2022
6. Tanaka N, Mori S, Kiyotani K, Ota Y, Gotoh O, Kusumoto S, Nakano N, Suehiro Y, Ito A, Choi I, Ohtsuka E, Hidaka M, Nosaka K, Yoshimitsu M, Imaizumi Y, Iida S, Utsunomiya A, Noda T, Nishikawa H, Ueda R, Ishida T. Genomic determinants impacting the clinical outcome of mogamulizumab treatment for adult T-cell leukemia/lymphoma. Haematologica, 107:2418-2431, 2022
7. Goda N, Sasada S, Shigematsu H, Masumoto N, Arihiro K, Nishikawa H, Sakaguchi S, Okada M, Kadoya T. The ratio of CD8 + lymphocytes to tumor-infiltrating suppressive FOXP3 + effector regulatory T cells is associated with treatment response in invasive breast cancer. Discover Oncology, 13:27, 2022
8. Kobayashi T, Kumagai S, Doi R, Afonina E, Koyama S, Nishikawa H. Isolation of tumor-infiltrating lymphocytes from preserved human tumor tissue specimens for downstream characterization. STAR protocols, 3:101557, 2022
9. Itahashi K, Irie T, Nishikawa H. Regulatory T-cell development in the tumor microenvironment. European journal of immunology, 52:1216-1227, 2022
10. Kelkka T, Tyster M, Lundgren S, Feng X, Kerr C, Hosokawa K, Huuhtanen J, Keränen M, Patel B, Kawakami T, Maeda Y, Nieminen O, Kasanen T, Aronen P, Yadav B, Rajala H, Nakazawa H, Jaatinen T, Hellström-Lindberg E, Ogawa S, Ishida F, Nishikawa H, Nakao S, Maciejewski J, Young NS, Mustjoki S. Anti-COX-2 autoantibody is a novel biomarker of immune aplastic anemia. Leukemia, 36:2317-2327, 2022
11. Nagaharu K, Kojima Y, Hirose H, Minoura K, Hinohara K, Minami H, Kageyama Y, Sugimoto Y, Masuya M, Nii S, Seki M, Suzuki Y, Tawara I, Shimamura T, Katayama N, Nishikawa H, Ohishi K. A bifurcation concept for B-lymphoid/plasmacytoid dendritic cells with largely fluctuating transcriptome dynamics. Cell reports, 40:111260, 2022
12. Aoki C, Imai K, Mizutani T, Sugiyama D, Miki R, Koya Y, Kobayashi T, Ushida T, Iitani Y, Nakamura N, Owaki T, Nishikawa H, Toyokuni S, Kajiyama H, Kotani T. Molecular hydrogen has a positive impact on pregnancy maintenance through enhancement of mitochondrial function and immunomodulatory effects on T cells. Life sciences, 308:120955, 2022
