Annual Report 2022
TEAM MAKINOSHIMA
Hideki MAKINOSHIMA
Introduction
During carcinogenesis and cancer progression, homeostasis, which maintains a constant biological state, is disrupted. Beyond the homeostatic mechanism, metabolites such as nucleic acids that induce carcinogenesis and metabolic systems specific to cancer cells are overproduced or decreased within individuals, and the metabolites themselves contribute to the development and malignant transformation of cancer. At the time of carcinogenesis or cancer progression, homeostasis, which keeps the state of the living body constant, is broken. We hypothesized that metabolites that induce carcinogenesis and the metabolic system unique to cancer cells are overproduced or reduced in individuals, and that the metabolites themselves contribute to the development and malignancy of cancer beyond its homeostatic mechanism. We are analyzing cancer cells, tumor tissues and blood samples from the viewpoint of cancer metabolism, especially focusing on nucleic acid metabolism.
The Team and What We Do
In FY2022 as well, we aimed to identify metabolites and metabolic pathways specific to cancer and to discover new drugs targeting them. Collaborative research between the Institute for Advanced Biosciences, Keio University and the National Cancer Center, collecting samples from patient-derived samples, cancer cell lines, cancer stromal cells, and mouse models, and analyzing the metabolome of cancer gone. In drug discovery research targeting metabolic pathways characteristic of cancer, we studied the regulatory mechanisms of nucleic acid biosynthetic pathways. Furthermore, we proposed the possibility of a new therapeutic method that inhibits the new biosynthetic pathway of purine nucleic acids with antifolates and the recycling pathway with new drugs.
Through collaborative research with Dr. Takahiro Yamauchi of the Department of Hematology and Oncology at the University of Fukui, blood samples were collected before the start of chemotherapy, the day after the start, and at the end of treatment. Metabolome analysis of these samples was performed using CE-MS (capillary electrophoresis-mass spectrometry) and GC-MS (gas chromatography-mass spectrometry), and metabolite profiles before and after chemotherapy were constructed. In CE-MS, 510 metabolites were analyzed, and quantitative values were obtained for 138 metabolites. Among them, 26 metabolites were statistically significantly changed (Figure 1). With GC-MS, 475 metabolites were analyzed, 262 metabolites could be measured, and 29 metabolites showed statistically significant changes (Figure 2). There were 17 metabolites that were commonly changed by both GC-MS and CE-MS methods.
Figure 1. Changes in 26 metabolites and uric acid-related metabolites analyzed by CE-MS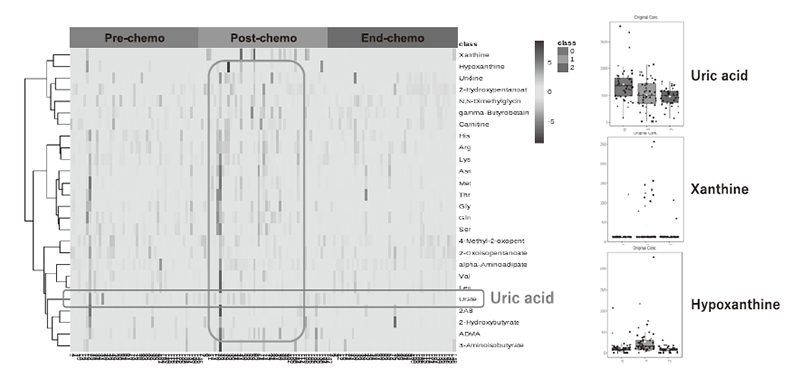

Figure 2. Changes in 29 metabolites and uric acid-related metabolites analyzed by GC-MS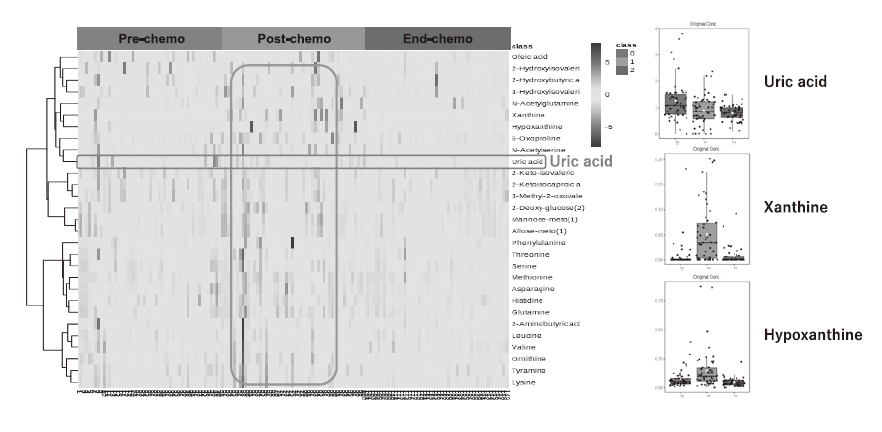

Research Activities
Some papers were published as the results of the above research activities. There is a paper on a screening system for drugs that inhibit cell motility using zebrafish embryos (Paper #1), and a paper on cinnamon, a Chinese herbal medicine, which inhibits glucose metabolism and suppresses cancer cell motility (Paper #2).
1. Gastrulation Screening to Identify Anti-metastasis Drugs in Zebrafish Embryos.
Nakayama J, Makinoshima H, Gong Z.
Bio Protoc. 2022 Oct 5;12(19):e4525. PMID: 36313195
2. Cinnamon bark extract suppresses metastatic dissemination of cancer cells through inhibition of glycolytic metabolism.
Nakayama J, Konno Y, Maruyama A, Tomita M, Makinoshima H.
J Nat Med. 2022 Jun;76(3):686-692. PMID: 35445961
Education
Miyu Narita (Shonai Regional Industry Promotion Center, Research assistant)
Yuhei Tawaragi (Shonai Regional Industry Promotion Center, Research assistant)
Chihiro HOMMA (Keio University Institute for Advanced Biosciences, Special Research Student)
Future Prospects
In fiscal 2023, we will continue to elucidate the regulation mechanism of purine nucleic acid metabolism using HPRT1-deficient cells, and aim to publish it as a research paper. Furthermore, we will identify enzymes important in the metabolic control mechanism of pyrimidine nucleic acids and discover new therapeutic targets.
