Home > Organization > Divisions and Independent Research Units > Division of Carcinogenesis and Prevention > Viral Carcinogenesis and Prevention Group > Research Projects > Development of HPV-associated cancer prevention
Development of HPV-associated cancer prevention
HPV infects to the basal cells of the stratified squamous epithelium and establishes persistent infection within the basal compartment. HPV infection can persist more than a decade, leading to develop cancers. Aim of this project is to develop cancer prevention for HPV associated cancers by eradicating viruses from persistently infected tissues.
Elucidating molecular mechanisms for HPV persistence.
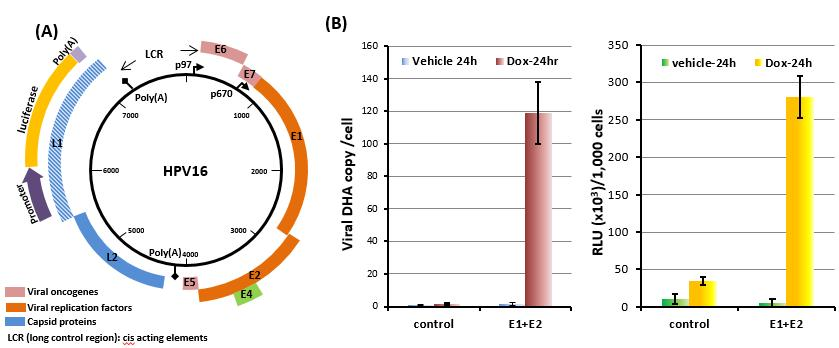
HPVs are small DNA viruses that contain an approximately 8,000bp double-stranded circular DNA as a genome (Fig.2A). The viral genomes are established as low copy number nuclear episomes by transient genome replication immediately following the virus infection (initial amplification) and then maintained at constant copy number in basal cells (maintenance replication). The massive increase of viral genome (productive amplification) and production of progeny virions occur exclusively in terminally differentiated, upper layers of the infected tissues(1).
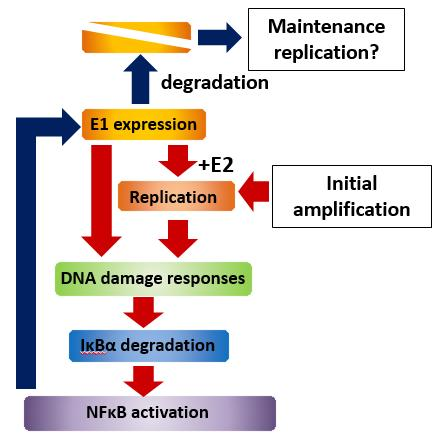
We have developed a tissue culture model for HPV persistent infection using normal human cervical keratinocytes (HCKs) immortalized in our laboratory and demonstrated that E1, an HPV encoded DNA helicase, is necessary for initial and productive amplification of HPV16 genome while it is dispensable for maintenance replication(2). Our recent study showed that ectopic expression of E1 induces activation of NFκB, a key regulator for immune and inflammatory responses, and NFκB in turn promotes proteasomal degradation of E1 in HCKs maintaining HPV16 genomes(3). We propose that this negative feedback loop is a regulatory mechanism to limit an E1 level and HPV replication in basal cells, critical to determine the viral persistence (Fig.1). Currently, we are further analyzing an E3 ubiquitin ligase complex responsible for E1 degradation and E1-independent mechanism of the viral maintenance in basal cells.
Figure 1. E1-NFκB negative feedback loop
Development of anti-viral drugs.
In order to exploit a high through-put screening for anti-viral drugs that target maintenance replication of HPVs, we have constructed and tested a several recombinant HPV16 genomes displacing a fragment of the viral genome with an expression cassette of a secreted luciferase. These novel HPV16 replicons were maintained at constant copy numbers, comparable with the wild type in HCKs and enabled highly sensitive monitoring of the maintenance replication (Fig.2B). We are preparing high through-put screenings to identify chemical compounds and/or host factors that eradicate persistent infection.
Figure 2