Home > Organization > Divisions and Independent Research Units > Division of Carcinogenesis and Prevention > Viral Carcinogenesis and Prevention Group > Research Projects > In Vitro Carcinogenesis of Human Cells
In Vitro Carcinogenesis of Human Cells
To clarify molecular mechanisms of oncogenesis by viral and cellular oncogenes and inactivation of tumor suppressors, we are establishing ex vivo carcinogenesis models for cervical cancer and other cancers by transducing abnormalities of genes found in cancer into normal cells-of-origin of each cancer.
HPV-induced carcinogenesis and its prevention
Most infections of HR-HPVs are inapparent and cleared by the host immune system. However, a small fraction of the infections can result in cancer development at various mucosa such as cervix and oropharynx. A critical event in neoplastic progression of cervix is upregulation of viral oncogenes, E6 and E7, in basal cells. In the viral life cycle, E6 and E7 levels are under tight control by the viral transcription factor, E2, thus, very limited in basal cells. Several mechanisms causing this upregulations of E6 and E7 have been reported such as the integration of the viral genome into host chromosomes with loss of E2 gene and methylation of the viral promoter which disables transcriptional suppression of E6 and E7 by E2. However, it is not entirely clear what may contribute to such disruption of the viral life cycle. We have established a human cervical keratinocyte cell line that harbors about 50 copies of episomal HPV16 genome. Using this tissue culture model for HPV infection, we covered that the viral helicase E1 is dispensable for the HPV genome maintenance and that E1 and NFκB forms a negative feedback loop to limit an E1 level and the viral replication in basal cells. It is possible that the disruption of this negative feedback loop can result in aberrant accumulation of E1 in basal cells. Ectopic expression of the E1 may cause abnormal replication of the viral genomes, constitutive activation of NFκB pathway. Currently, we are investigating significance of the disruption of the E1-NFκB feedback loop and neoplastic progression of precancerous cervical lesions. Recent genome editing technology with nucleases such as Zinc finger nuclease and CRISPR/Cas made it possible to directly target HPV genome whether or not it is integrated. With the CRISPR/Cas system, we are developing targeting vector to knock down E6/E7 regions of HPV16 and 18.
Human cancer xenograft model utilizing normal pancreatic duct epithelial cells
Pancreatic ductal adenocarcinomas (PDACs) are considered to arise through neoplastic transformation of human pancreatic duct epithelial cells (HPDECs). In order to evaluate the biological significance of genetic and epigenetic alterations in PDACs, we isolated primary HPDECs and established an in vitro carcinogenesis model. Firstly, lentivirus-mediated transduction of KRASG12V, MYC and human papillomavirus 16 (HPV16) E6/E7 under the control of a tetracyclin-inducible promoter efficiently immortalized and transformed primary HPDECs, which gave rise to adenocarcinomas subcutaneously in an immune-deficient mouse xenograft model, depending on expression of the four genes. The tumors regressed promptly upon shutting-off the oncogenes, and the remaining tissues showed histological features corresponding to normal ductal structures with simple columnar epithelium. Re-expression of the oncogenes resulted in development of multiple PDACs through pancreatic intraepithelial neoplasia-like structures (Fig 3). We also succeeded in efficient immortalization of primary HPDECs with transduction of mutant CDK4, cyclin D1 and TERT. In combination with p53 silencing, KRASG12V alone was sufficient to fully transform the immortalized HPDECs, and MYC markedly accelerated the development of tumors.
Figure 1. Ex vivo pancreatic carcinogenesis model

Acquisition of malignant traits in squamous cell carcinomas
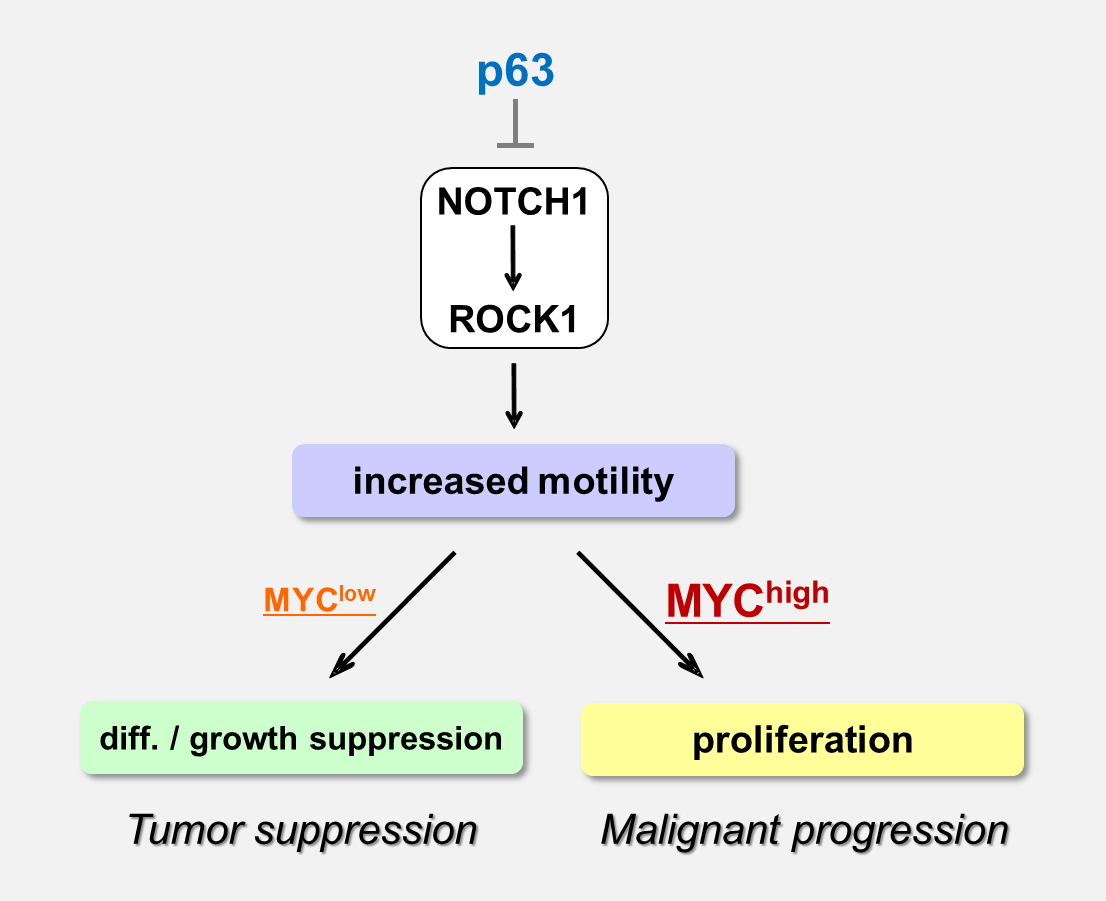
We have been studying the biological significance of p63 expression and the molecular basis underlying acquisition of malignant traits in squamous cell carcinomas (SCCs) of cervical and head and neck cancers. p63 is a master regulator for the development of stratified epithelium and has a role in stem cell maintenance. In line with its physiological role, p63 is frequently overexpressed in more than 50% of human SCCs including examples in the cervix, lung, and head and neck. Paradoxically, loss of p63 is shown to be associated with metastasis appearance and poor prognosis in SCCs, although the underlying molecular mechanism and its functional relevance to carcinogenesis remains unclear as is the case with NOTCH1. We have identified the novel NOTCH-ROCK pathway downstream of p63. Using our in vitro human multistep carcinogenesis model for cervical cancer, we revealed that knock-down of p63 conferred increased invasiveness through the NOTCH-ROCK pathway in MYC-overexpressing cells (Figure 4). We also confirmed the tendency of mutual exclusivity between p63 and MYC expressions in head and neck SCCs by TCGA analysis. We aim to develop novel strategies for the diagnosis and molecularly targeted therapy against poorly-differentiated SCCs based on its cancer biology.
Figure 2. Proposed model for the NOTCH-ROCK pathway and its biological significance
in squamous cell carcinomas

