Home > Information > press release > Breakthrough Discovery on the Structure and Activation Mechanism of ALK
Breakthrough Discovery on the Structure and Activation Mechanism of ALKInternational Collaborative Team of Ghent University and National Cancer Center Japan
14 October 2021
Ghent University
National Cancer Center Japan
in Japanese
Summary
Cells communicate with their environment via receptors on their surface. When a protein approaches these receptors, they can pass along a message to the inside of the cell, for example the instruction to grow which can lead to tumor formation. New research by the international collaborative team of Dr. Savvas Savvides from the Unit for Structural Biology, VIB-UGent Center for Inflammation Research (Ghent, Belgium) and Dr. Akihide Yoshimi from Cancer RNA Research Unit, Research Institute, National Cancer Center Japan (Tokyo, Japan; President: Hitoshi Nakagama) reveals the 3D structure of the ALK receptor, which is involved in various cancers and other diseases. These insights can lead to the understanding of the function of these receptors and the first important step towards therapeutic approaches.
This work is published in the prestigious journal Nature on 13 October 2021.
Background
Cells communicate with their environment via proteins that attach to receptors on the cell surface. Think of these receptors as communication antennas that can only receive specifically encoded signals. When an appropriate signal from outside the cell – in the form of a specific protein for the receptor – approaches the antenna, the signal will be communicated to the inside of the cell. This can initiate many important cellular processes, such as cell growth and division.
When the communication between such proteins and receptors becomes uncontrolled, excessive, or interrupted, this can lead to severe diseases such as cancers, inflammation, and autoimmune disorders. A group of receptors central to human health is known as the receptor tyrosine kinases (RTKs). We have about 58 of these RTKs organized into 20 families. Functional defects in these receptors are relevant for several cancers, autoimmune diseases, neurological diseases, and metabolic disorders.
Discoveries and authors’ comments
Due to the critical roles of RTKs in physiology and disease, most RTKs have been well-researched. However, the structural and functional roles of one of the RTK groups, the ALK family, have not been understood well. We have known about the ALK family receptors for over three decades, and yet structural information about how they interact with their signaling proteins remained to be clarified.
This new study has revealed the 3D structure of ALK and LTK (another ALK family RTK), as well as their structures when bound to their activating proteins. This is critical information for understanding the function of these receptors.
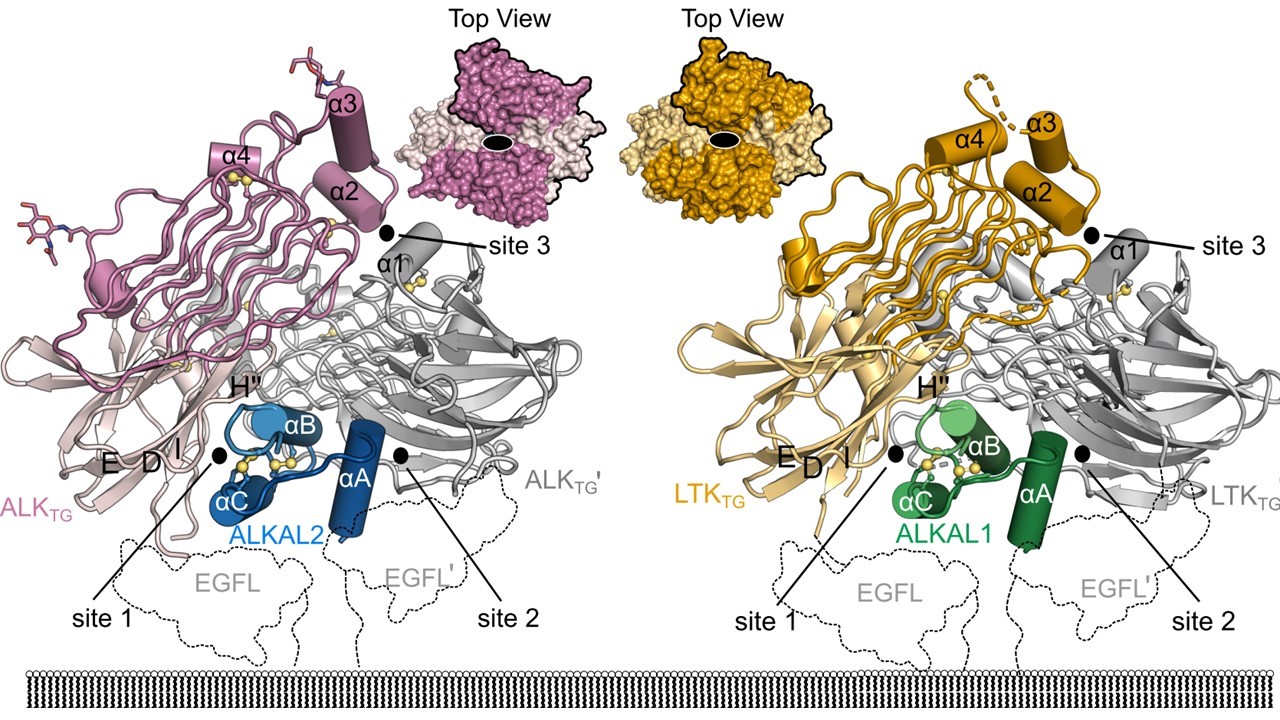
Figure: Structures of the assembly of ALK receptor (“antenna”) with ALKAL2 (“signal” protein or cytokine) (left) and LTK receptors with ALKAL1 (right).
Steven De Munck: "We were surprised by how unique and unprecedented these proteins and their assemblies are, illustrating how basic research is essential for uncovering novel concepts and mechanisms in disease-causing processes."
ALK and its mutated versions are involved in multiple disorders including neuroblastoma, colon cancer, melanoma, and possibly obesity. However, the lack of structural insight has prevented the specific therapeutic targeting of ALK receptors.
Savvas Savvides emphasizes the impact of their finding: "Frontline research in structural biology is vital for addressing difficult problems in biology, no matter how challenging such research might appear to be. It takes a team of talented and dedicated scientists to achieve breakthroughs like this, which will inspire innovative therapeutic approaches and open new biomedical questions.”
Specific mutations in ALK, which are found in a variety of cancers as above, may affect the structure of the cytokine-receptor interfaces and increase cytokine binding to the ALK receptor.
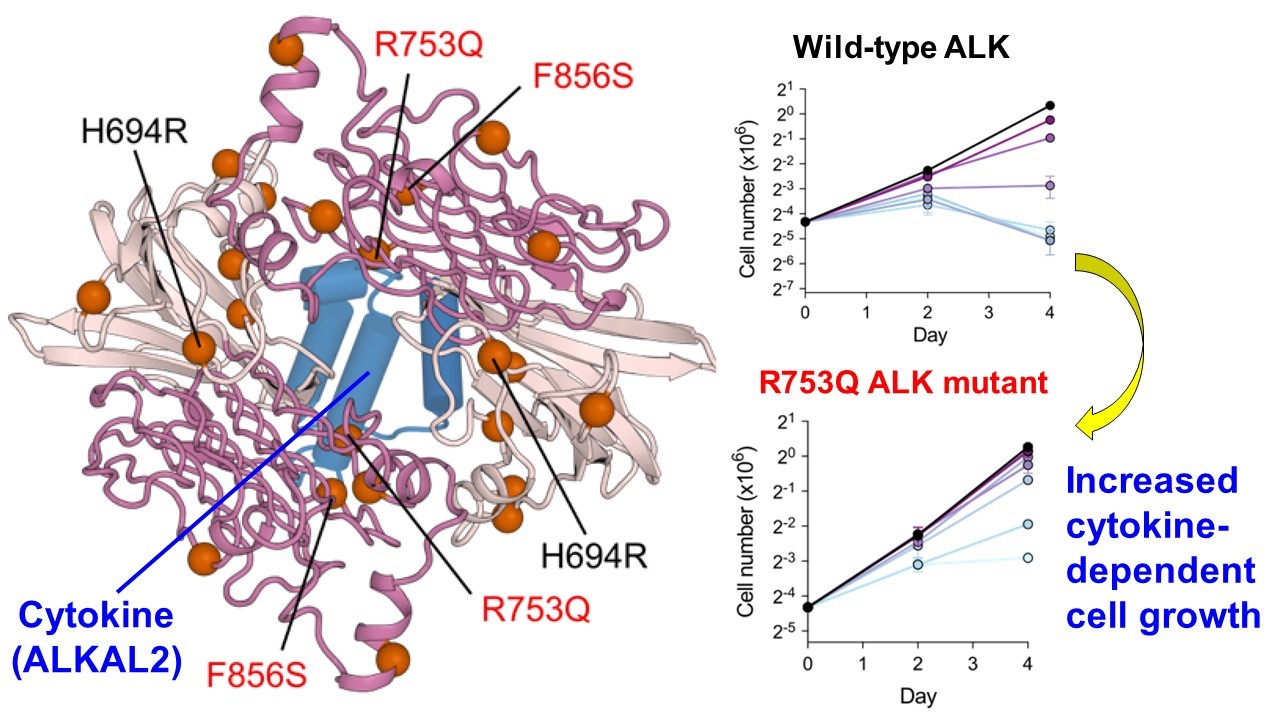
Figure: The two variants of ALK (R753Q and F856S) are equidistant from the cytokine (ALKAL2) (left), suggesting that these mutations affect the binding affinity of cytokine to ALK. Consistent with this, cells bearing these mutations have dramatically increased cytokine-dependent cell growth.
Akihide Yoshimi: “In this international collaborative study, both experts in structural biology from Belgium and cancer biologists from National Cancer Center Research Institute, Japan tackled the structural basis for the activation of ALK family receptors as well as the mechanistic and biological roles of the mutant forms of ALK that are found in multiple types of cancers. Such an interdisciplinary effort enabled us to achieve this breakthrough, which will open an avenue to develop novel therapeutics targeting the ALK family receptors.”
Publication
Journal
Nature
Title
Structural basis of cytokine-mediated activation of ALK family receptors
Authors
Steven De Munck, Mathias Provost, Michiko Kurikawa, Ikuko Omori, Junko Mukohyama, Jan Felix, Yehudi Bloch, Omar Abdel-Wahab, J. Fernando Bazan, Akihide Yoshimi, Savvas N. Savvides
Publication date
13 October 2021
DOI
10.1038/s41586-021-03959-5
URL
https://www.nature.com/articles/s41586-021-03959-5(link to external site)
Support
This study was partly supported by the following grants awarded to Dr. Akihide Yoshimi.
- Home-Returning Researcher Development Research (grant number 19K24691) from the Japan Society for the Promotion of Science (JSPS)
- Grand-in-aid for Scientific Research (A) (KAKENHI grant number 21H04828) from JSPS
- National Cancer Center Research and Development Funds (grant number 2020-A-2)
Contact information
Regarding the paper’s content
Section Head: Akihide Yoshimi
Cancer RNA Research Unit, Research Institute
National Cancer Center Japan
5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045, Japan
phone: +81-3-3542-2511
E-mail: ayoshimi●ncc.go.jp
https://www.ncc.go.jp/en/ri/division/cancer_rna/index.html
Media inquiries
Office of Public Relations, Strategic Planning Bureau, National Cancer Center Japan
5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045, Japan
phone: +81-3-3542-2511
fax: +81-3-3542-2545
E-mail: ncc-admi●ncc.go.jp

