Home > Information > press release > MASTER KEY ASIA
A New Asia-Pacific CollaborationMASTER KEY ASIAPromoting genomic medicine throughout Asia
October 20, 2021
National Cancer Center, Japan
in Japanese

Summary
- “MASTER KEY Asia” has launched, a prospective international registration study for rare cancers with ten partner facilities within the Asia-Pacific including Malaysia, Thailand, Indonesia, Philippines, and Vietnam.
- The first academic participants are from Malaysia and the Philippines, with whom processes such as external quality assurance of patients’ samples are shared, with a central pathological review, and genomic analysis conducted in Japan.
- By accumulating data to the largest rare cancer database worldwide, MASTER KEY Asia will accelerate clinical development and genomic medicine throughout Asia. Genomic information, treatment details and prognosis of rare cancer patients will be aggregated to promote international multicenter clinical trials.
The "MASTER KEY Asia Project," an international collaboration promoting rare cancers research and development throughout Asia including Malaysia, Thailand, Indonesia, Philippines, and Vietnam, led by the Hospital (NCCH; director: Kazuaki SHIMADA) of the National Cancer Center (president: Hitoshi NAKAGAMA) is launched.
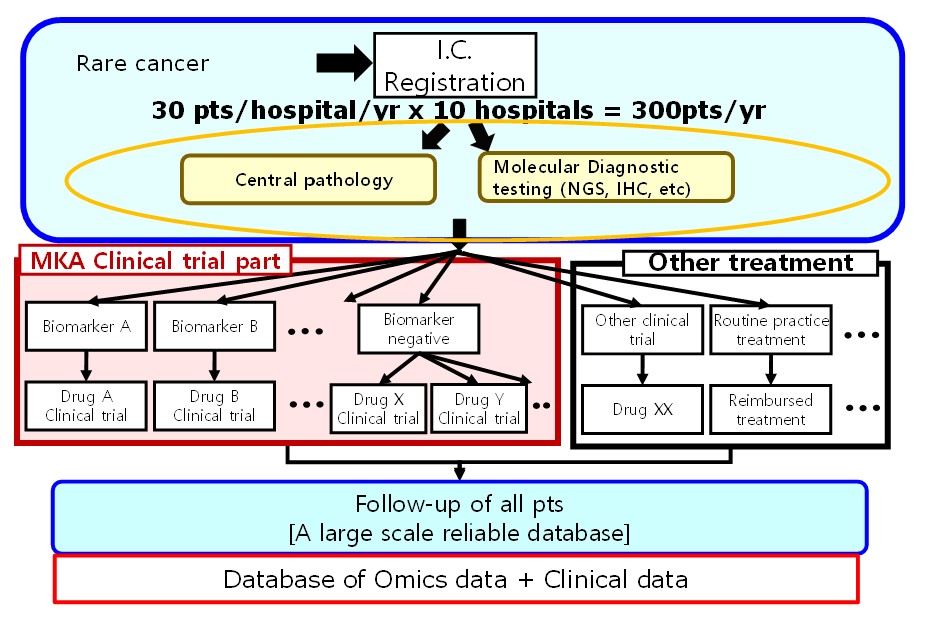
This project builds onto the “MASTER KEY Project (Japan)” launched in 2017, for driving research and development for rare cancers and for promoting genomic medicine, a successful collaboration between industry and academia. It has already enrolled over 2000 patients in Japan. We have now opened this registry to Asian partners. Genomic information, treatment details, and prognoses will be aggregated to a large-scale database, comprising the registry arm. It also has an arm for conducting investigator/industry initiated trials with rare cancer patients.
Genomic medicine for cancer patients is not yet common in Asia. With this project, genomic information from Next Generation Sequencing (NGS), together with treatment details, and prognoses will be collected. MASTER KEY Project (Japan) had 2107 patients registered as of September 2021, the largest database worldwide for rare cancers. 12 pharmaceutical companies have joined in the study, 19 clinical trials have been conducted (12 investigator-initiated, 7 pharmaceutical-sponsored). With the expansion, enrollment of 1000 patients per year from Asia including Japan are anticipated.
Collaborating with Asian countries, with which more genomic characteristics are shared as opposed to western countries, and leveling up of cancer genomic medicine in the region is indispensable to driving discoveries for Asian patients and to deliver Asian-specific effective treatments to rare cancer patients.
Having developed the gene panel test “OncoGuideTM NCC Oncopanel System” through the "TOP-GEAR project" and followed through with its implementation with advanced medical care, and establishing a center dedicated to rare cancers, NCCH has launched a project for establishing a clinical trials network for Asia, ATLAS project in 2020. MASTER KEY Asia will work in sync with ATLAS on developing new drugs for rare cancers.
Background
Rare cancers are defined as "malignant tumors with an incidence of less than 6 per 100,000 people per year". Rare cancers comprise nearly 200 cancer types for example, soft tissue sarcoma, GIST, pediatric cancers, brain tumors, eye tumors, skin cancer, head and neck cancers, malignant mesothelioma, cancer of unknown primary, neuroendocrine tumors and hematological cancers. Due to its rarity, standard treatments are yet to be established unlike common cancers, and the prognoses remain poorer than for common cancers.
Rare cancers altogether account for about 22% of all cancer patients. With half of cancer patients worldwide anticipated from Asia, there is a pressing need to invite Asian countries to participate in clinical trials for developing treatments for rare cancers. With smoking rates remaining high, implementation of cancer screening and genomic medicine still in initial stages, we wish to support building the infrastructure for clinical research and clinical trials throughout Asia.
MASTER KEY Asia
“Personalized medicine” is introduced with testing of genomic alterations, guiding molecular targeting therapies. However, these studies with NGS analysis focus on common cancers, limiting studies of rare cancers. Moreover, most clinical trials are based in Europe or the US, there is limited biological information focusing on Asian populations.
The "MASTER KEY Project" targets progressed rare cancer, cancer of unknown primary and rare subtypes of common cancers, consisting of two major arms. The first is a registry study that comprehensively collects genetic and clinical information, prognostic data, etc. of patients with rare cancers and constructs a large-scale database (registry part). The second comprises new generation clinical trials called “basket trials” (sub-study part) based on specific biomarker tests. Launched in 2017 by NCCH, four partner institutions have joined the scheme, recording a larger number of patient registrations than hoped for.
With “MASTER KEY Asia,” clinical information of ever more rare cancers will become available, accelerating research of target variants and treatment development.
We will work in sync with the Asian clinical TriaLs network for cAncerS project:ATLAS project, to establish a clinical trial infrastructure in Asia whilst advancing clinical research, preparing to tackle the exponential growth in Asia.
MASTER KEY Project
July,31st 2017 press release
https://www.ncc.go.jp/jp/information/pr_release/2017/0731/index.html
MASTER KEY project Web site
https://www.ncc.go.jp/jp/ncch/masterkeyproject/index.html
MASTER KEY Asia
PI Noboru YAMAMOTO
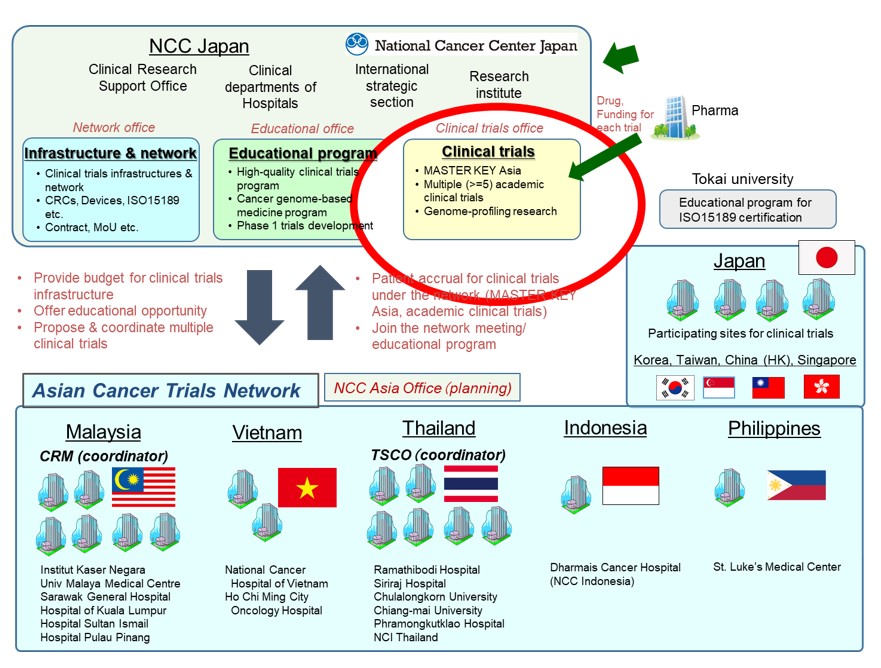
Participating Sites
Malaysia
- Sarawak General Hospital
- Hospital Sultan Ismail
- Hospital Kuala Lumpur
- National Cancer Institute
- Hospital Pulau Pinang
- University Malaya Medical Center
Thailand
- Ramathibodi Hospital
Indonesia
- Dharmais Hospital
Philippines
- St Luke’s Medical Center
Vietnam
- Ho Chi Minh City Oncology Hospital
- National Cancer Institute
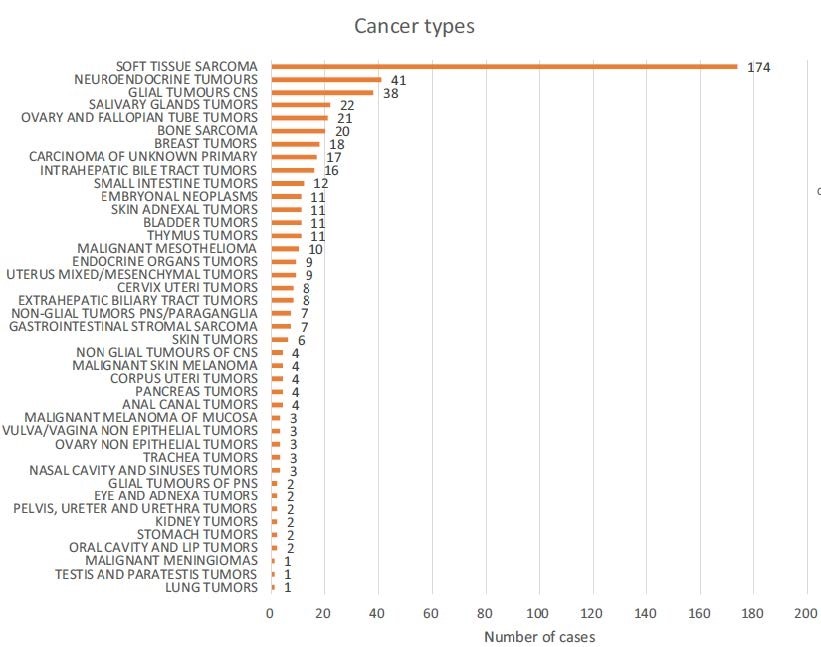
Figure1 Cancer types included in MASTER KEY
ATLAS Project
The ATLAS project aims to build an Asian cancer clinical trials network with medical institutions where multiple registration-directed trials can be conducted on a constant basis, by means of reinforcing international collaborative research operations at NCCH and developing clinical trial infrastructure at ASEAN countries. The project is mainly for investigator-initiated registration-directed trials, but investigator-initiated non-registration trials and industry-sponsored trials can also utilize this platform. Improving cancer patients’ drug access and implementing genomic medicine is our mission, solving Asia specific issues by Asians. The initiative is anticipated to benefit the entire Asian region, by enrolling more patients in clinical trials, thus speeding up the process of developing new drugs.
Sep 9th, 2020 Press Release
https://www.ncc.go.jp/jp/information/pr_release/2020/0909/index.html
Message from participating sites
Message from St. Luke’s Medical Center (Philippines) Dr Marcelo Imasa
The current direction of cancer therapeutics is the delivery of treatment that is tailored to the unique genomic alterations in the tumor and its microenvironment, which vary between cancer types and between patients. By recognizing these differences, therapies against cancer can be delivered with precision for each individual patient. Most of the studies on genomic alterations and precision medicine, however, are focused on the more common cancers, with majority of patient data coming from the Caucasian population. MASTER KEY ASIA bridges the gap in genomic information on rare cancers particularly among the Asian population.
As ethnic, socio-economic, environmental, and even cultural diversity may impact cancer behavior, its characteristics, and eventually its management, MASTER KEY ASIA creates a framework of collaborative international research that may enable delivery of precision medicine in the context of diversity. This comes at an opportune time when the disparity in cancer treatment outcomes is now even further heightened by the inequity of patients’ access to new technologies that offer hope of improved survival. We, at St. Luke’s Medical Center together with our Filipino cancer patients, are thus grateful for this privilege to collaborate with the National Cancer Center Hospital, the NCC Research Institute, and our colleagues from the ASEAN on the MASTER KEY ASIA PROJECT.
Message from Hospital Sultan Ismail (Malaysia) Dr Syafirin
We would like to extend our sincere appreciation to the Japan Government and National Cancer Centre Hospital (NCCH) for recruiting Hospital Sultan Ismail (HSI) Oncology team to be a part of the Asian Clinical Trials Network for Cancers (ATLAS). The HSI Oncology team has been involved in many clinical trials locally and internationally. We hope this is a stepping stone to many more collaborations in cancer research.
This research provides a platform to study and understand the cancer genomics in rare cancers. And therefore, helps towards the development of new oncology drugs and advancement in cancer genomic medicine. This is a collaboration of multi-national research centres and we believe this project will contribute to the development of cancer treatment in the future. We take great pleasure in being a part of this research collaboration and contributing towards cancer treatment development.
We are here to better understand and to help tackle some of the biggest questions in the field of Oncology. Through MASTER KEY Asia, we hope to contribute and provide better cancer care in the future especially in the treatments of rare cancers.
Message from Sarawak General Hospital (Malaysia) Dr Lim
I would like to express my most sincere gratitude to National Cancer Center Hospital (NCCH) initiatives in strengthening the clinical research network across Asia through the launching of the Asian Clinical Trials Network for Cancers (ATLAS). Sarawak General Hospital is honoured to be part of this Network. In this collaboration, we have the opportunity to participate in the MASTER-KEY project, and further build up our research capacity and infrastructures required for cancer genomic medicine and early Phase 1 drug development.
Cancer is one of the most complex highly heterogeneous diseases, it is common to see differences in the progression or therapeutic response of the same tumour type. Understanding the growth and development of human tumours using mathematics and biological data is a burgeoning area of cancer research. Ten years ago, lung cancer was classified as either small cell or non-small cell, but it is now described by the presence or absence of nearly 30 genetic mutations. Each cancer-causing or cancer-influencing genetic mutation that is discovered is a potential target for drug development, cancer genomics data could also potentially provide insights into how an individual’s cancer might progress, and its likely response to treatment.
The MASTER-KEY project will definitely enhance our cancer-genomic database in rare cancers, and support the translation of those data into new treatments options. Lastly, we truly appreciate again all the opportunities and guidance from NCCH and we are very excited to learn from your esteemed team.
Message from Sarawak General Hospital (Malaysia) Dr Muthukkumaran Thiagarajan
Hospital Kuala Lumpur’s Department of Radiotherapy and Oncology has long aspired to be a leading institution in cancer research in Malaysia.
We are truly honoured to establish partnership with National Cancer Centre Hospital in Japan in conducting the ATLAS project, which is a gateway for clinical trial and research opportunities. This partnership is expected to improve human resource development in cancer research and assist in building infrastructure in-within our institution to expand research initiatives. The opportunity to participate in international clinical trials is also truly exciting.
We hope that our collaborative efforts will ensure better access of our patients to the state of the art treatment strategies and elevates the care provided to our population of cancer patients.
Message from Hospital Pulau Pinang (Malaysia) Dr Soo Hoo
A collaboration to improve research activity in cancer care and to bring drug development to a molecular and genetic approach, MASTER-KEY will open up doors of research for ASEAN countries. A heartiest congratulations to National Cancer Center Hospital for this leadership!
Funding
This project is funded by Japan Agency for Medical Research and Development (AMED).
Contact
MASTER KEY Project Registry / clinical trials
Department of International Clinical Development,
National Cancer Center Hospital, Tokyo
Hitomi OKUMA
5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045
E-mail:NCCH2007_office●ml.res.ncc.go.jp
Others
Office of Public Relations, Strategic Planning Bureau
E-mail:ncc-admin●ncc.go.jp
Files.
Links.
- ATLAS Project (Link to External Site.)
- MASTER KEY Project Overview

