Home > Information > press release > Approximately 43,000 individual patient data of clinical trials with colorectal cancer in Asia and the West shared
Approximately 43,000 individual patient data of clinical trials with colorectal cancer in Asia and the West shared―ARCAD Database Project has established new international data-sharing framework promoting R&D in cancer medicine―
August 4, 2022
National Cancer Center Japan
The ARCAD Foundation
Mayo Clinic
In Japanese
Key points of the announcement
- National Cancer Center Hospital East (“Hospital East”), the ARCAD Foundation, and Mayo Clinic have established a new data-sharing platform in the “ARCAD Database Project“, consisting of individual patient data (IPD) of approximately 43,000 metastatic colorectal cancer (mCRC) patients.
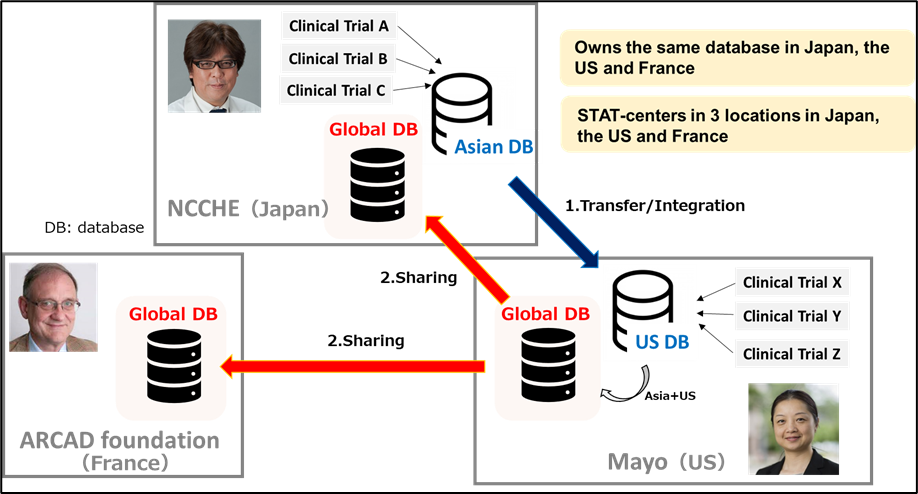
- Currently, a total of 56 trial owners shared the IPD with ARCAD Foundation, with 50 trials including 42,095 IPD from international/wester trials transferred to Mayo Clinic, and six trials including 974 IPD from Asian trials transferred to Hospital East. The integrated ARCAD Database is mirrored at three statistics and data centers (SDCs).
- Following previous successes, an innovative research portfolio will be developed to build evidence through international collaboration and to apply the IPD to promote cancer R&D to deliver optimal treatment to as many patients as possible.
Overview
National Cancer Center Hospital East (Japan), the ARCAD Foundation (France), and Mayo Clinic (United States) have established a new data-sharing platform in the “ARCAD Database Project”, consisting of individual patient data (IPD) of approximately 43,000 metastatic colorectal cancer (mCRC) patients. Currently, the ARCAD Database Project is conducted jointly by Prof. Aimery de Gramont (The ARCAD Foundation), Prof. Qian Shi (Mayo Clinic) and Prof. Takayuki Yoshino (Hospital East).
On June 30, Hospital East transferred six trials including IPD of 974 Asian patients into the ARCAD Database. Mayo Clinic, in charge of the data-management of the mCRC ARCAD Database, has integrated the 974 Asian IPD to the master database. Then, the integrated ARCAD Master Database was mirrored at all three statistics and data centers (SDC) at Mayo Clinic shared with Hospital East, the ARCAD Foundation, and Mayo Clinic, with 56 trials including IPD of approximately 43,000 patients.
In the late 2000s in the West, the ARCAD Foundation launched “ARCAD Database Project,” a database project to integrate clinical studies data on mCRC. On September 15, 2021, the National Cancer Center and the ARCAD Foundation signed the collaborative agreement. Since September 2021, Hospital East has been running “ARCAD Asia (*1)”, a data-sharing project to collect and integrate completed clinical trial IPD mostly from Asia to apply it to areas such as drug R&D.
Going forward, details of the ARCAD Database Project will be evaluated to see how the databases should be used and applied, including international collaborations with academia. The project will be further spurred toward developing a system to build evidence, based on international collaboration.
Comment from Professor Aimery de Gramont (The ARCAD Foundation):
“The ARCAD Clinical Trials Program was launched in June 2007 to develop international guidelines for new methods of evaluating colorectal cancer treatments. This is a major project, extremely ambitious and with a major international impact.
This program has been renamed mCRC ARCAD Database. Currently, more than 90 experts from all over the world are actively involved in the completion of this project, constituting the ARCAD Group, an international scientific committee. This committee meets twice a year in the United States and Europe respectively. These experts contribute voluntarily to the publication of "Position Papers", documents composed of several annual articles published in international peer-reviewed journals and thus aiming at changing the methodology of therapeutic trials.
In early 2020, we were delighted to set a new collaborative partnership with the National Cancer Center Hospital East in Japan, under the leadership of Professor Takayuki Yoshino, allowing the inclusion of new studies from the Asian continent in this database and the opening of a third statistical analysis center, ARCAD Asia Data Center.
The National Cancer Center Japan is an internationally renowned center of excellence and one of the most active and productive in clinical research. The fact that it has agreed to endorse and integrate the ARCAD Asia Data Center into its fold is an undeniable guarantee of the quality of the work to come and the support to be expected from industry and governments on the Asian continent, while preserving the independence and autonomy of the ARCAD Database.”
Comment from Professor Qian Shi (Director of Biostatistics Shared Resource, Mayo Clinic Comprehensive Cancer Center):
“Mayo Clinic statistical team has collaborated with Prof. de Gramont and the ARCAD Foundation since the inception of the mCRC ARCAD Database. It was original lead by Dr. Daniel J. Sargent, one of the world’s foremost experts in biostatistics and oncology who brought together disparate investigators and established data sharing across academia and industry internationally. Following Dan’s footsteps, I am honored to continue Dan’s legacy. Having been working on mCRC ARCAD database since 2008, and recently with colleagues from National Cancer Center Hospital East, I feel so fortunately to be able to collaborate with so many talented oncologist, surgeons, statisticians, and other trialists. It is also very exciting to see that many clinically impactful publications in prestigious journals and international conferences were based on the database that we helped put together!
Personally, I think it is revolutionary that Asian trials joined the ARCAD. In a sense, the ARCAD database is truly international now! Working side-by-side, with Japanese statistical team, on continuous ARCAD database integration, we are greatly impressed by Japanese colleagues’ expertise and dedications. I and Mayo team are looking forward to the new collaborations and adventure with both the ARCAD Foundation and ARCAD Asia Data Centers”
Comment from Dr. Takayuki Yoshino (Deputy Director/Head, Department of Gastrointestinal Oncology, National Cancer Center Hospital East):
“I am glad that the ARCAD has been expanded to Asia. By including data on Asian IPD into the ARCAD Database, it will be possible to accurately examine racial differences using the IPD. It also has the potential to create an opportunity for Asian researchers to grow into global leaders. We strive to grow the ARCAD Database Project into larger and comprehensive Database that includes other cancer types, beyond mCRC Database.”
Projections
The ARCAD Database Project bolsters worldwide building of evidence and development of cancer treatment through various analyses based on extensive data from world-class clinical trial and studies databases on colorectal cancer. It will also systematically assess issues from the perspective of regulatory science (*2), making suggestions about end points (*3) in clinical trials and studies, and in regulatory processes, through international cooperation. Its aim is also to develop personnel who will be global leaders through international collaborations among academia.
Overview of the institution
National Cancer Center Japan (https://www.ncc.go.jp/en/index.html)
Established in 1962 as a national agency, the National Cancer Center Japan has been spearheading advanced research as a pivotal national organization on cancer research and cancer treatment.
National Cancer Center Hospital East, established in 1992, is one of the best designated cancer hospitals in Japan, visited by over 9,000 new patients a year. With the mission of providing world-class cancer treatment and creating new cancer treatments, the hospital has been designated as a Clinical Research Core Hospital and a Cancer Genome Medicine Core Hospital of Japan. As the R&D hub based on international networks, and together with the adjacent Exploratory Oncology Research & Clinical Trial Center, the National Cancer Center Japan has been developing state-of-the art drugs and medical devices for cancer, promoting leading-edge personalized treatment including genome medicine, with many successes.
The ARCAD Foundation (https://www.fondationarcad.org/en)(linked at extenal site)
The ARCAD Foundation was founded in 2006 for charitable purposes by Professor Aimery de Gramont. The foundation engages in wide-ranging initiatives inside and outside of France, including gastrointestinal cancer research and patient support programs.
This is the paragraph we would like to put describing the ARCAD Foundation:
ARCAD Foundation is a publicly recognized foundation, its legal status granted in 2006 by the French Government, created by its Chairman-Founder Pr Aimery de Gramont, engaging in wide-ranging initiatives inside and outside of France, with three major objectives :
- make the general public and health workers sensitive to the need for prevention and early screening of digestive cancers, thus raising awarness
- promote improvement of patient care and clinical research in the area of digestive cancers
- provide better information and support to patients suffering from digestive cancers.
Mayo Clinic (https://www.mayoclinic.org/about-mayo-clinic)(linked at extenal site)
Mayo Clinic is a nonprofit organization committed to clinical practice, education and research, providing expert, whole-person care to everyone who needs healing. Mayo Clinic’s mission is to inspire hope and contribute to health and well-being by providing the best care to every patient through integrated clinical practice, education, and research. Our primary value is “The needs of the patient come first.” Mayo Clinic is top-ranked for quality more often than any other health care organization. Once again, Mayo Clinic in Minnesota has been recognized as the best hospital in the nation for 2022-2023 by U.S. News & Wold Report.
Mayo Clinic Comprehensive Cancer Center (https://www.mayoclinic.org/departments-centers/mayo-clinic-cancer-center)(linked at extenal site)
More than 150,000 people with cancer come to Mayo Clinic each year. They find experts with extensive experience in the diagnosis and treatment of virtually every kind of cancer and the resources to provide excellent care tailored to their needs. Mayo Clinic doctors and researchers solve the most serious and complex medical questions, one person at a time. U.S. News & World Report consistently ranks Mayo Clinic among the top hospitals for cancer in the nation.
The Mayo Clinic Comprehensive Cancer Center is designated by the National Cancer Institute as a comprehensive cancer center. This means the clinic's renowned physicians, researchers and scientists carry out team-based, patient-centered research to develop the latest technologies and treatments to address unmet patient needs. As a result, people who come to the clinic for cancer care have access to hundreds of clinical trials in all phases.
Mayo Clinic researchers share what they learn with other members of the National Comprehensive Cancer Network, a not-for-profit alliance of leading cancer centers dedicated to improving the quality, effectiveness and efficiency of care for cancer patients.
Terminology
*1 ARCAD Asia (Aide et Recherche en CAncérologie Digestive Asia)
“ARCAD Asia Management Office Data Center” has been established within Hospital East, signing a joint research agreement on data provision with pharmaceutical companies, developing a data-sharing structure within the consortium. The project is currently ongoing, with the sharing being started for clinical trial and studies data on unresectable, advanced recurrent (stage 4) colorectal cancer, with a plan to include frequently seen cancer types in Asia in the future. As of July 2022, ARCAD Asia is in agreements with providers of data such as corporations and academia to share data of approximately 11 clinical trials and 2,974 cases of colorectal cancer, which are currently being integrated.
◆Members (as of July 2022):
Atsushi Ohtsu (Director, National Cancer Center Hospital East,); Yoshihiko Maehara (Professor Emeritus of Kyushu University, Director, Kyushu Central Hospital of the Mutual Aid Association of Public School Teachers); Takayuki Yoshino (Deputy Director/Head, Department of Gastrointestinal Oncology, National Cancer Center Hospital East); Eiji Oki (Associate Professor, Department of Surgery and Science, Graduate School of Medical Sciences, Kyushu University); Kentaro Yamazaki (Chief, Division of Gastrointestinal Oncology and Clinical Trial Coordination Unit, Shizuoka Cancer Center); Hideaki Bando (Assistant chief, Department of Gastrointestinal Oncology/Chief, Division of Drug and Diagnostic Development Promotion, National Cancer Center Hospital East); Masanori Terashima (Deputy Director, Shizuoka Cancer Center); Kohei Shitara (Assistant chief, Department of Gastrointestinal Oncology, National Cancer Center Hospital East)
◆Reference
September 30, 2021 press release
Spearheading data-sharing environments in Asia to lead the world’s R&D: International clinical trial data-sharing project “ARCAD Asia” launches
https://www.ncc.go.jp/jp/information/pr_release/2021/0930/index.html
ARCAD Asia website: https://www.ncc.go.jp/jp/ncce/division/arcadasia/index.html
*2 Regulatory science
A science for making accurate, evidence-based forecasts, evaluations and judgments with the purpose of applying results from sciences and technologies for the good of people and society, adjusting such results to the most ideal form possible for harmony between people and society.
*3 End-point
Indicator for evaluating the efficacy and safety of drugs and other factors in clinical studies.
Contact
On Project
National Cancer Center Japan
Mai Itagaki, Head, Section of Research administration
ARCAD Asia Management Office Data Center
TEL: +81-4-7133-1111 (main line)
Email: arcadasia_office●east.ncc.go.jp
The ARCAD Foundation
Dr Lama SHARARA, Director general, CEO
4 Rue kléber, 92300, Levallois-Perret, France
TEL: + 33 1 47 31 69 19
Email: lama.sharara●fondationarcad.org
Mayo Clinic
Dr. Qian Shi, Professor of Biostatistics and Oncology
Mayo Clinic, 200 First Street SW, Rochester, MN 55905, USA;
Tel: +1 507-538-4340
Email: shi.qian2●mayo.edu
From the press and media
National Cancer Center Japan
Office of Public Relations, Strategic Planning Bureau (Kashiwa Campus)
Email: ncc-admin●ncc.go.jp
