Home > Organization > Division of Translational Genomics(Tsukiji) > Research Summary
Research Summary
Establishment of an NGS-based genomic testing system
We developed an NGS-based in-house genomic testing system (NCC Oncopanel) to identify somatic gene mutations, amplifications, and fusions using an original cancer gene panel (NCC oncopanel), and have been continuously improving this system to become a clinically useful in vitro diagnostics (IVD) system in cooperation with the Department of Clinical Genomics and the Department of Bioinformatics of the National Cancer Center Research Institute (NCCRI). In cooperation with staff of the Department of Pathology and Clinical Laboratories in the National Cancer Center Hospital (NCCH), the SCI-Lab was opened in the NCCH, where our clinical sequencing system is operated under an international standard quality assurance. 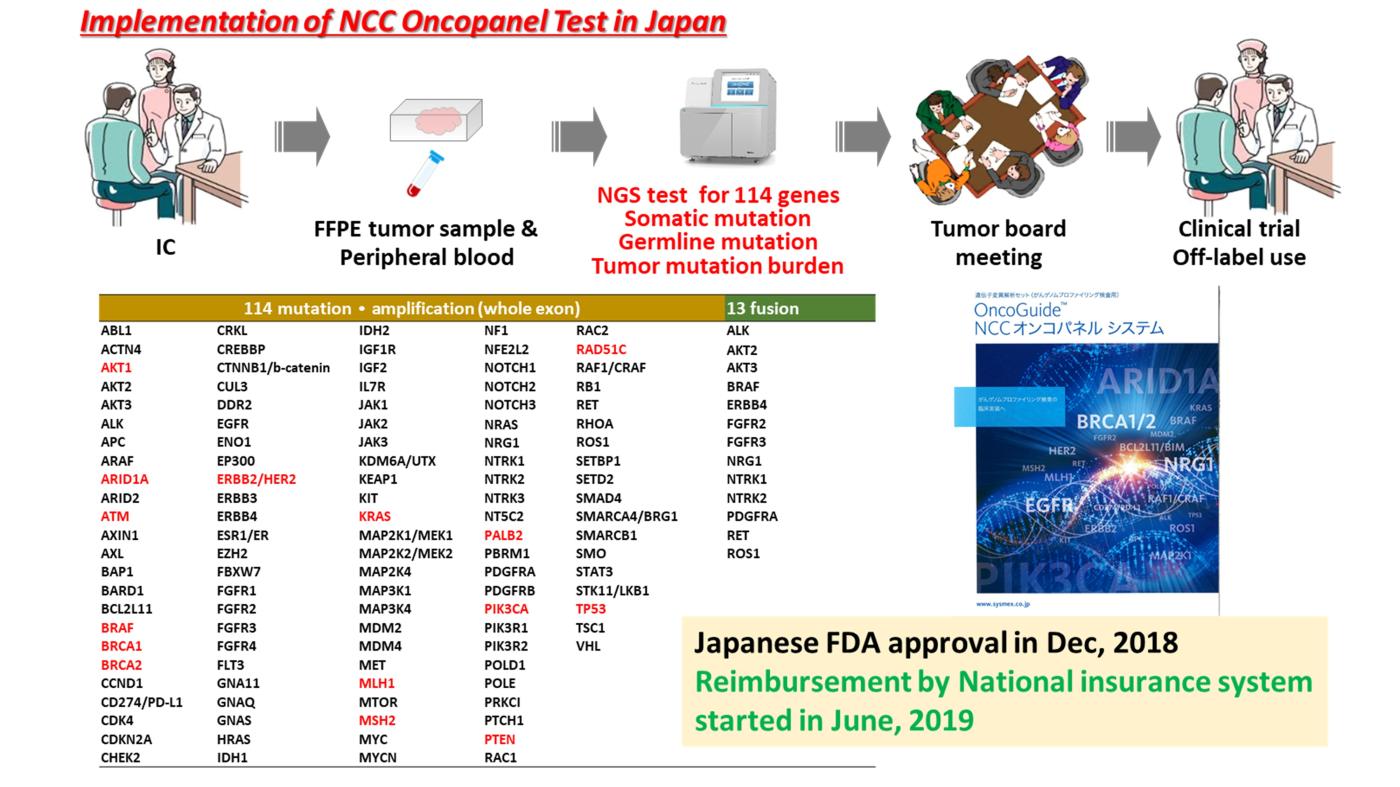
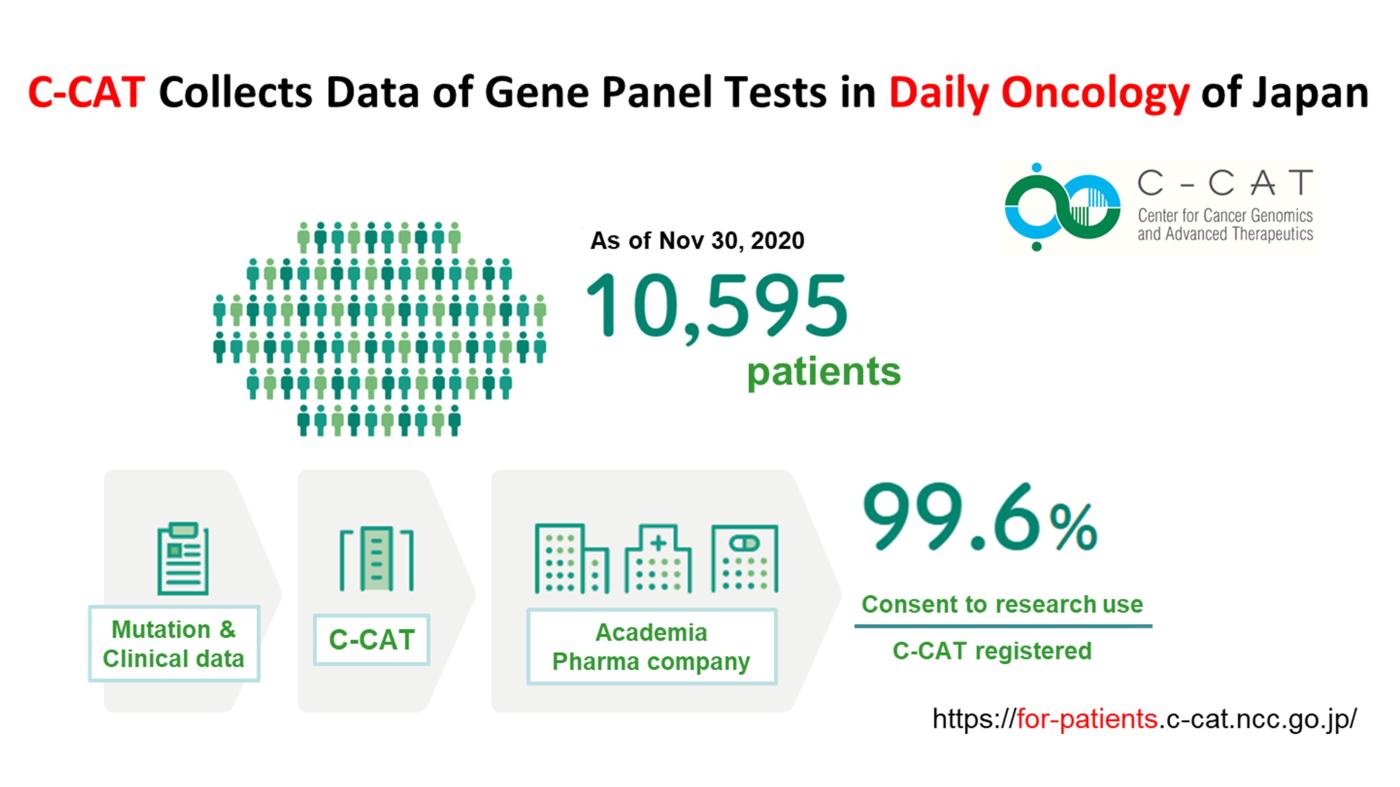
The NCC Oncopanel system was approved by PMDA as the first tumor-profiling gene panel tests in Japan on December 25, 2018, and and its use is reimbursed by the National Health Insurance system of Japan. Mutation and clinical data of the patients undergoing gene panel testing are collected by Center for Cancer Genomics and Advanced Therapeutics (C-CAT) of National Cancer Center Japan. As of Nov 2020, data of more than 10,000 patients with many kinds of cancer were collected. More than 99% of patients consented to the use of the collected data for research purposes. The C-CAT data will be shared with academic and industry researchers this year (https://for-patients.c-cat.ncc.go.jp/registration_status/).
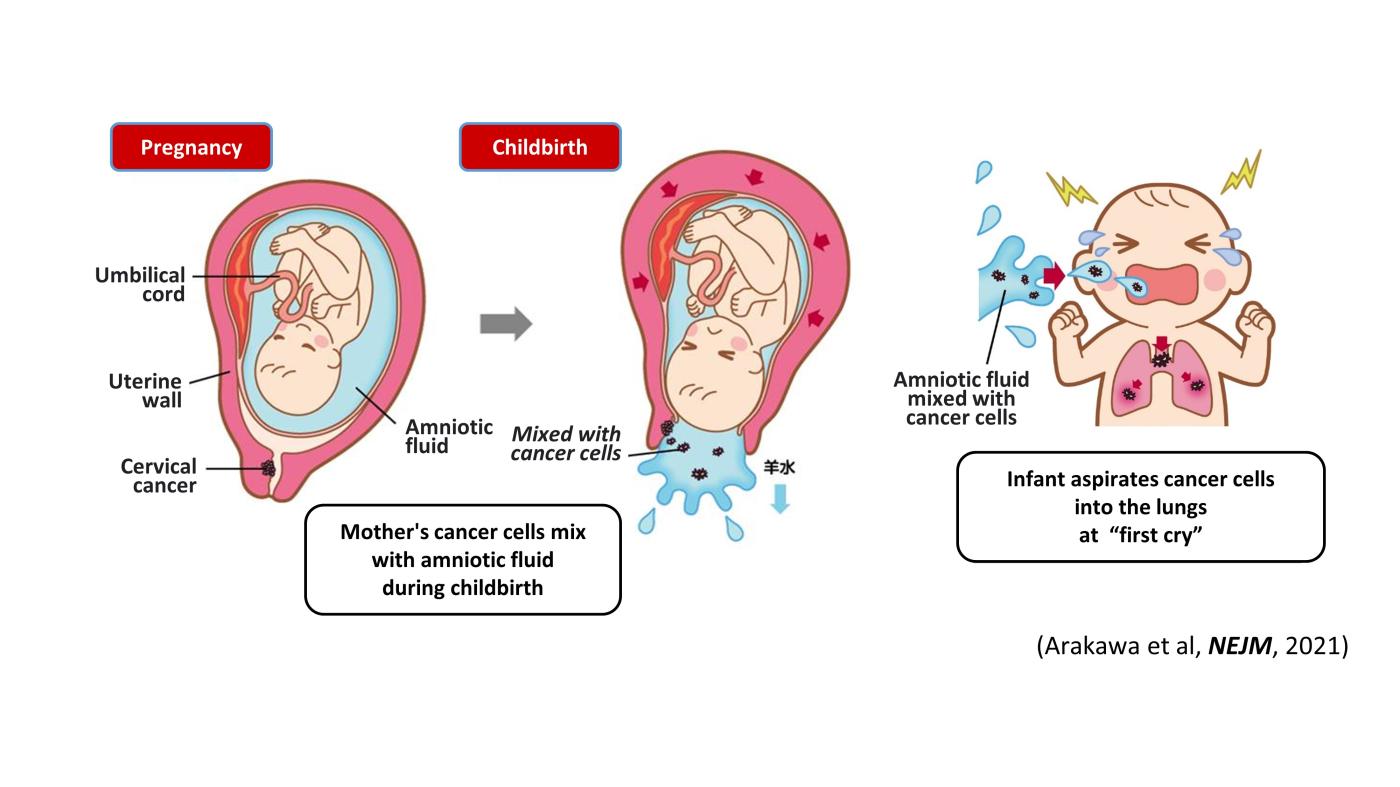
Two cases of pediatric lung cancer (in 23-month-old and 6-year-old boys) resulting from mother-to-infant transmission of uterine cervical tumors were incidentally detected during routine next-generation sequencing (NCC Oncopnale test) of paired samples of tumor and normal tissue. Spontaneous regression of some lesions in the first child and slow growth of the tumor mass in the second child suggested the existence of alloimmune responses against the transmitted tumors. Immune checkpoint inhibitor therapy with nivolumab led to a strong regression of all remaining tumors in the first child (Arakawa et al, NEJM, 2021).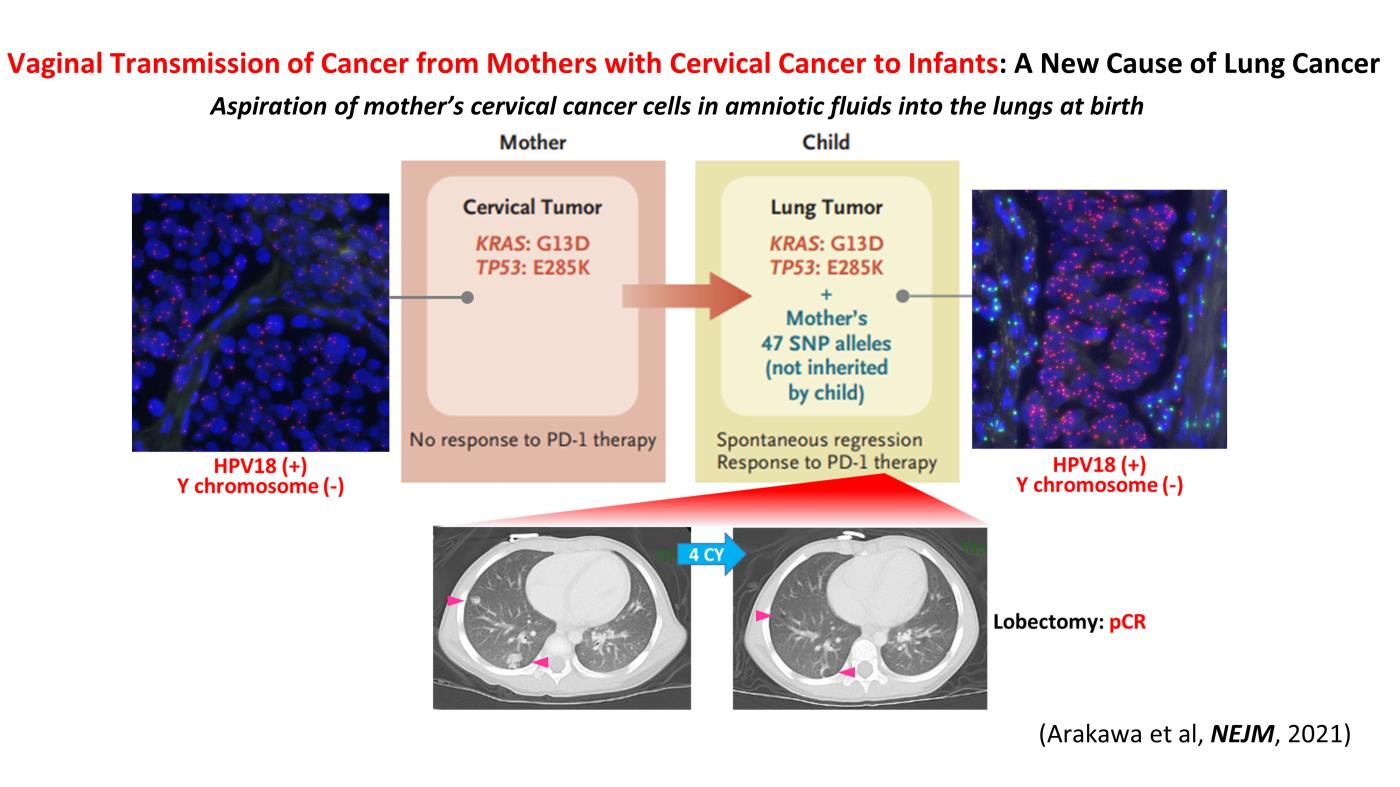
Implementation of NGS-based genomic testing in the NCCH
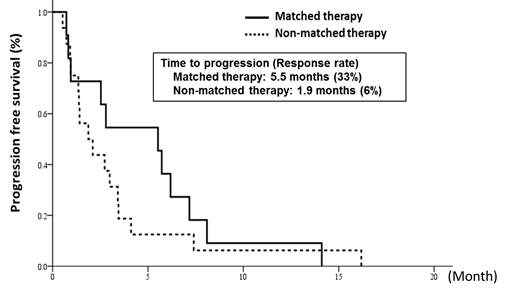
In cooperation with staff of the Department of Experimental Therapeutics and the Department of Pathology and Clinical Laboratories in the NCCH, prospective clinical studies were performed to examine the feasibility and utility of our NGS-based genomic testing system for patients enrolled into early-phase clinical trials. The result of the TOP-GEAR project (1st stage) study, in which patients were enrolled in 2013 and 2014, indicated that patients who received investigational drug therapies matched to their genomic aberrations showed better progression-free survivals than those who received non-matched therapies (Tanabe et al, Mol Cancer, 2016).
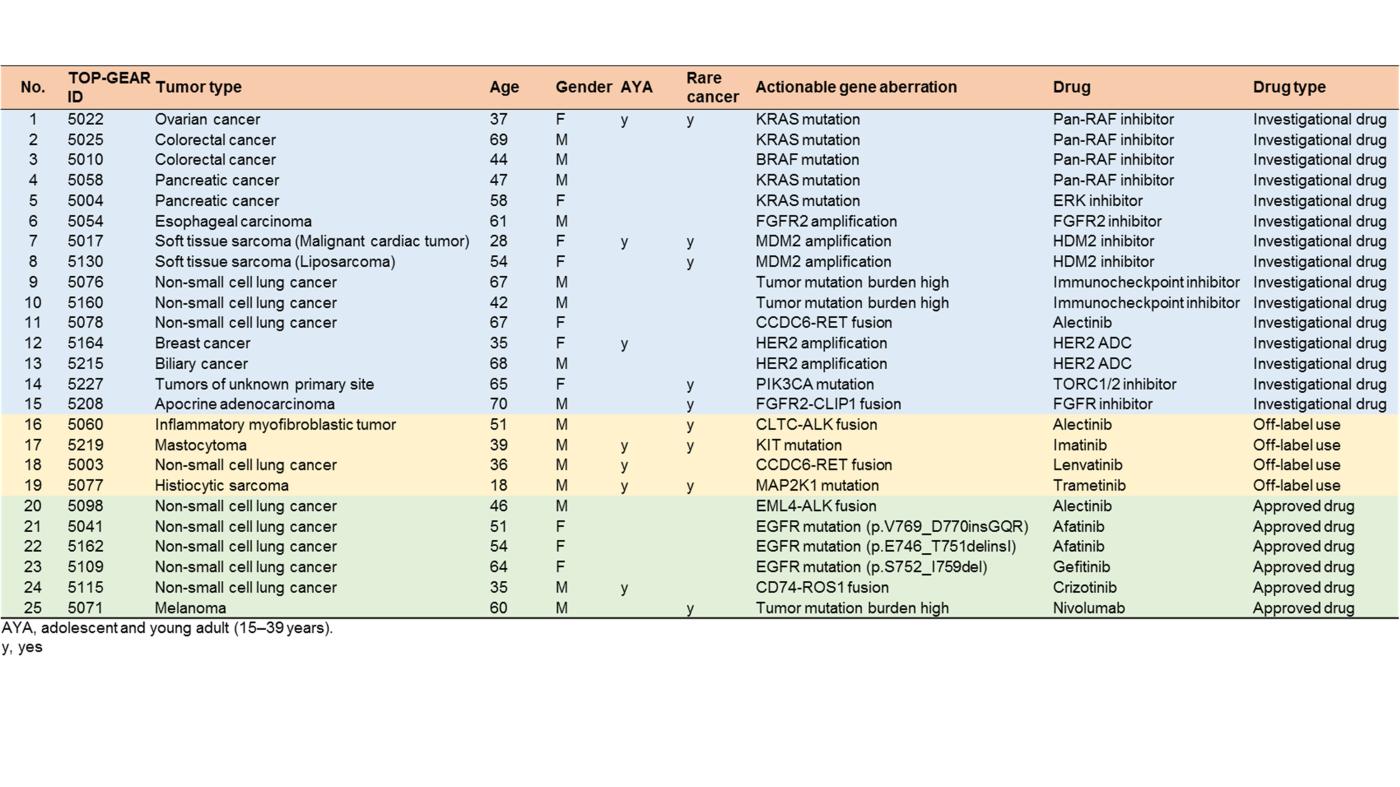
In 2016-2017, we performed a hospital-based prospective study (TOP-GEAR project, 2nd stage) to investigate the feasibility and utility of NGS-based analysis of 114 cancer-associated genes (the NCC Oncopanel test). We examined 230 cases (comprising > 30 tumor types) of advanced solid tumors, all of which were matched with non-tumor samples. Gene profiling data were obtained for 187 cases (81.3%), 111 (59.4%) of which harbored actionable gene aberrations according to the Clinical Practice Guidelines for Next Generation Sequencing in Cancer Diagnosis and Treatment (Edition 1.0) issued by three major Japanese cancer-related societies. Twenty-five (13.3%) cases have since received molecular-targeted therapy according to their gene aberrations. These results demonstrated the utility of tumor-profiling multiplex gene panel testing in a clinical setting in Japan. This study is registered with UMIN Clinical Trials Registry (UMIN000011141) (Sunami et al, Cancer Sci, 2019).
Based on these results, NCC Oncopanel has now been approved by PMDA (Pharmaceuticals and Medical Devices Agency; Japanese FDA) and reimbursed by National Health Care system in Japan (from June 1st, 2019).