Home > Information on C-CAT Data Utilization > Contents/Research-Use Portal
Contents/Research-Use Portal
About C-CAT Research-Use Portal site
“C-CAT Research-Use Portal site” is a portal site to utilize data accumulated in C-CAT.
- Combination search is available for clinical and gene mutation information of all C-CAT registered patients nationwide, who gave consent for the research use of their data by third-party investigators, and the search results can be browsed and downloaded
- Available data include all gene mutation (including germline variants) from CGP and clinical information (excluding ID’s).
- Hospital ID and registration ID are also available upon approval from the C-CAT Data Utilization Review Board (Registration ID is displayed only for members of Hospitals for Cancer Genomic Medicine upon approval).
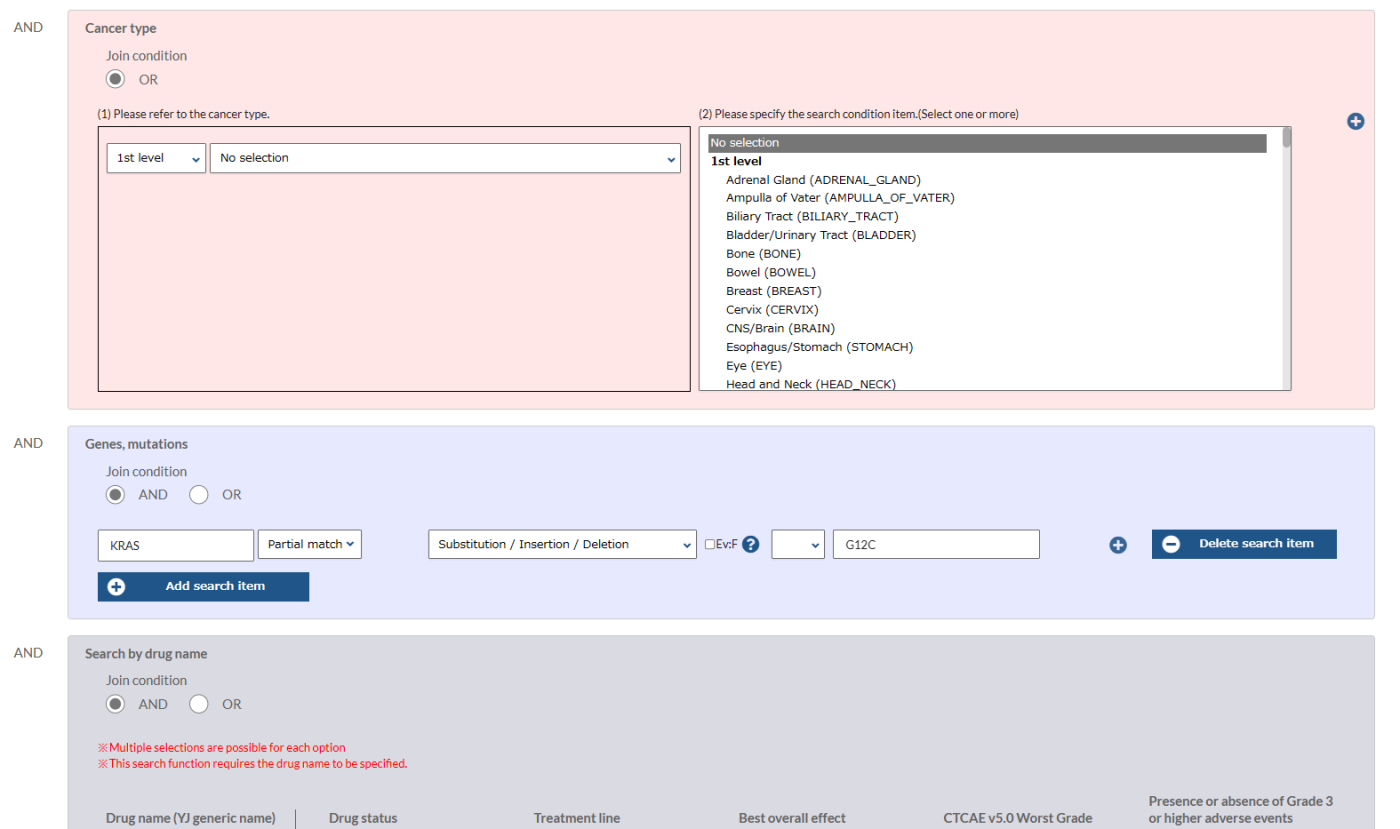
Search Conditions
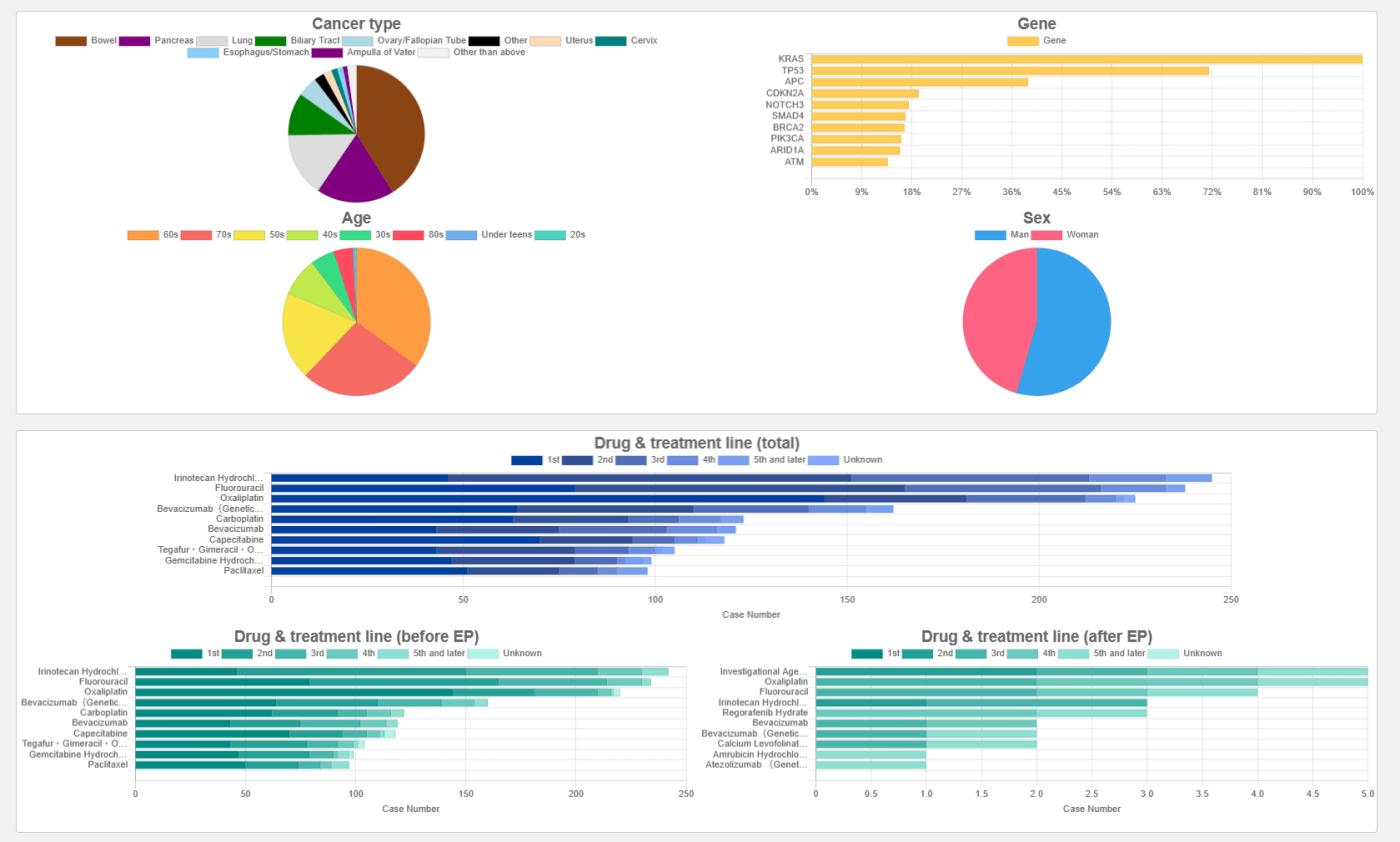
Search result data and graphs
To use the C-CAT Research-Use Portal site, you may need first a review and approval from Research Ethics Committee (REC) and head of your institution, unless your research proposal is outside the scope of the ethical guidelines (such as a market research purpose). Then the application form of the C-CAT data usage is to be submitted for a review by the C-CAT Data Utilization Review Board, and once approved, the data access licensing contract is required. Please refer to “Steps from application to data utilization” for details.
Information to be provided
With the patient's consent, stored data are available for the purpose of academic research or pharmaceutical development.
Comprehensive Genome Profling (CGP) test has started in Japan in June 2019 as a part of the healthcare services provided by the national health insurance system. The CGP data and clinical information of the patients are collected and stored at C-CAT upon individual informed consent. Data of the patients who further agreed that their data can be used by third-party investigators are available for research/investigation in both the academic and industrial sectors for the purpose of academic research or pharmaceutical development [Informed consent: Item 7]. Any result/intellectual property obtained from the use of the C-CAT data belongs to the user.
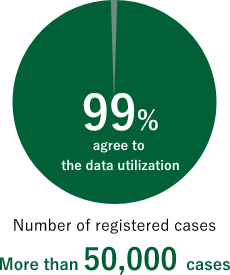
More than 50,000 cases registered, and more than 99% of them agree to the data utilization.
The number of the patients registered in C-CAT exceeded 10,000 in November 2020. As of the end of March 2023, more than 50,000 patients have been registered. Over 99% of the patients have agreed the data utilization by third-party researchers. Please refer to C-CAT registration status (external website) for the latest registration status.
Clinical information
| Basic patient information |
Hospital (Facility)ID, Sex, Age, Cancer type, etc. |
|---|---|
| Sample Information |
Inspection type, Tumor cell content, Specimen collection site, etc. |
| Background information |
Pathological diagnosis, Smoking history, ECOG-PS, family history, etc. |
| Cancer type Information |
Presence/absence of metastasis, companion diagnostic test results, etc. |
| Pre-/Post- EP Regimen information |
Drug name, Start/End date of administration Best overall response, Adverse events, etc. |
| Outcome information |
Outcome, Last survival confirmation date, Date of death, Cause of death |
Review by C-CAT Date Utilization Review Board
-
Hospitals for Cancer Genomic
Medicine Hospitals*Free of charge
-
Industries
*Free for use for publicly Funded researches
-
Research Institutes
*Paid
- Cancer type
- Genetic mutations reported in CGP tests
- Drug name
- Therapeutic response, Adverse event
Use search results for research and development
Contact for inquiries
Section of Data Science Strategy Center for Cancer Genomics and Advanced Therapeutics (C-CAT), National Cancer Center
5-1-1 Tsukiji, Chuo-ku, Tokyo, 104-0045, Japan
E-mail:E-mail : c-cat_use●ml.res.ncc.go.jp (change ● to @)
