トップページ > 研究組織一覧 > 分野・独立ユニットグループ > 希少がん研究分野 > 研究プロジェクト > 希少がん研究の基盤構築 > PDC(Patient-derived Cell)・PDX(Patient-derived Xenograft)の樹立 更新
PDC(Patient-derived Cell)・PDX(Patient-derived Xenograft)の樹立 更新
PDC (Patient Derived Cell)とPDX(Patient-Derived Xenograft)の樹立
PDC (Patient-Derived Cells)やPDX(Patient-Derived Xenograft)はがん研究に必須のモデルとして使われてきました。たとえば、新しく発見した遺伝子異常の機能的な意義や、新しい薬の薬効評価などにおいて、PDCやPDXは使われています。しかしながら、希少がんではPDCやPDXは一般に入手がきわめて困難です。たとえば、肉腫を例に挙げると、公的な細胞バンクやバイオバンクに保管されているPDCやPDXはごく一部の肉腫にすぎません。また、細胞バンクに保管されている従来のPDCには、臨床情報が付随していない、遺伝的背景の情報がない、現在の診断基準とは異なる基準で診断名がついている、などの問題があります。なにかの研究をしようと思ったときにすぐに立ちはだかるのが、適切なPDCやPDXがない、という問題です。同様のことは肉腫以外のほとんどの希少がんについてあてはまります。「疾患として定義されてから何十年も経つにもかかわらず細胞株が一つもない」というのが、希少がんのよくある状況です。こういったことが原因となって、治療法の開発が進まないのです。
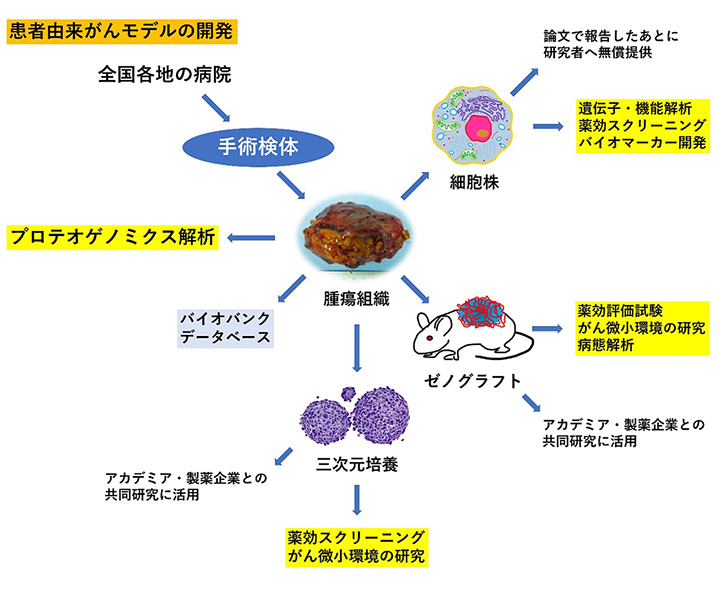
希少がん研究分野は、腫瘍組織からPDCやPDXの樹立を行っています。樹立されたPDCやPDXの分子背景の解析を行い、分子標的薬の標的となる遺伝子の異常を同定したり、希少がんに適応拡大可能な抗がんを探索したりしています。また、効率よく細胞株を樹立する方法を確立しています。樹立した細胞株は論文として発表し研究者に提供し、企業との共同研究に活用しています。
図 手術検体から細胞株、ゼノグラフトを作成する流れ。新しい三次元培養法を構築中です。
希少がんのPDCやPDXにご興味のある方はご一報ください。
文献
Kondo T. Current status and perspectives of patient-derived rare cancer models. Hum Cell. 2020 Jun 14. doi: 10.1007/s13577-020-00391-1. Epub ahead of print. PMID: 32537685. [PubMed](外部リンク)
提供可能な肉腫細胞株
2024.12.04更新
|
Sarcoma type |
Cell line name |
Cellosaurus ID |
|
骨巨細胞腫 |
NCC-GCTB1-C1 |
CVCL_A9QC |
|
NCC-GCTB2-C1 |
CVCL_A0SR |
|
|
NCC-GCTB3-C1 |
CVCL_A0SS |
|
|
NCC-GCTB4-C1 |
CVCL_B3VJ |
|
|
NCC-GCTB5-C1 |
CVCL_C1GF |
|
|
NCC-GCTB6-C1 |
CVCL_C2WT | |
|
NCC-GCTB7-C1 |
CVCL_C2WX | |
|
NCC-GCTB8-C1 |
CVCL_D4C0 | |
|
NCC-GCTB9-C1 |
CVCL_D4C1 | |
| NCC-GCTB10-C1 | Not available | |
| NCC-GCTB11-C1 | Not available | |
| NCC-GCTB12-C1 | Not available | |
| NCC-GCTB13-C1 | Not available | |
| NCC-GCTB14-C1 | Not available | |
| NCC-GCTB15-C1 | Not available | |
|
粘液線維肉腫 |
NCC-MFS1-C1 |
CVCL_VT41 |
|
NCC-MFS2-C1 |
CVCL_A2EI |
|
|
NCC-MFS3-C1 |
CVCL_B3RM |
|
|
NCC-MFS4-C1 |
CVCL_B3RL |
|
|
NCC-MFS5-C1 |
CVCL_B5YX |
|
|
NCC-MFS6-C1 |
CVCL_D4AD | |
|
NCC-MFS7-C1 |
Not available | |
|
NCC-MFS8-C1 |
Not available | |
|
悪性末梢神経鞘腫 |
NCC-MPNST1-C1 |
CVCL_YU12 |
|
NCC-MPNST2-C1 |
CVCL_YU13 |
|
|
NCC-MPNST3-C1 |
CVCL_YU14 |
|
|
NCC-MPNST3-X2-C1 |
CVCL_YU15 |
|
|
NCC-MPNST4-C1 |
CVCL_YU16 |
|
|
NCC-MPNST5-C1 |
CVCL_YU17 |
|
|
NCC-MPNST6-C1 |
CVCL_B4H4 |
|
|
隆起性皮膚線維肉腫 |
NCC-DFSP1-C1 |
CVCL_UR53 |
|
NCC-DFSP2-C1 |
CVCL_UR54 |
|
|
NCC-DFSP3-C1 |
CVCL_ZE79 |
|
|
NCC-DFSP4-C1 |
CVCL_D1GH |
|
|
NCC-DFSP5-C1 |
CVCL_D4ZK |
|
|
脱分化型脂肪肉腫 |
NCC-DDLPS1-C1 |
CVCL_A1BV |
|
NCC-DDLPS2-C1 |
CVCL_A5IJ |
|
|
NCC-DDLPS3-C1 |
CVCL_A6FB |
|
|
NCC-DDLPS4-C1 |
CVCL_B3TQ |
|
|
NCC-DDLPS5-C1 |
CVCL_B7EM |
|
|
NCC-DDLPS6-C1 |
CVCL_C1GH |
|
|
滑膜肉腫 |
NCC-SS1-C1 |
CVCL_VG70 |
|
NCC-SS2-C1 |
CVCL_VE84 |
|
|
NCC-SS3-C1 |
CVCL_ZC97 |
|
|
NCC-SS4-C1 |
CVCL_A6FC |
|
|
NCC-SS5-C1 |
CVCL_C1GG |
|
|
NCC-SS6-C1 |
CVCL_E3AB |
|
|
未分化多形肉腫 |
NCC-UPS1-C1 |
CVCL_B3TP |
|
NCC-UPS1-X3-C1 |
CVCL_VR16 |
|
|
NCC-UPS2-C1 |
CVCL_RP62 |
|
|
NCC-UPS3-C1 |
CVCL_B3RN |
|
|
NCC-UPS4-C1 |
CVCL_B6KI |
|
|
平滑筋肉腫 |
NCC-LMS1-C1 |
CVCL_LK56 |
|
NCC-LMS1-X3-C1 |
CVCL_VM07 |
|
|
NCC-LMS2-C1 |
CVCL_A4EE |
|
|
NCC-LMS3-C1 |
CVCL_D4A9 | |
|
CIC再構成肉腫 |
NCC-CDS1-X1-C1 |
CVCL_YL54 |
|
NCC-CDS1-X3-C1 |
CVCL_YL55 |
|
|
NCC-CDS2-C1 |
CVCL_YL70 |
|
|
骨肉腫 |
NCC-OS1-X2-C1 |
CVCL_VM08 |
|
NCC-ESOS1-C1 |
CVCL_YD21 |
|
|
NCC-OS2-C1 |
Not available |
|
|
低悪性線維粘液肉腫 |
NCC-LGFMS1-C1 |
CVCL_B0W9 |
|
粘液型脂肪肉腫 |
NCC-MLPS1-C1 |
CVCL_A1VS |
|
NCC-MLPS2-C1 |
CVCL_C1GI |
|
|
NCC-MLPS3-C1 |
CVCL_C1GJ |
|
|
胞巣状軟部肉腫
|
NCC-ASPS1-C1 |
CVCL_ZZ81 |
|
NCC-ASPS2-C1 |
CVCL_E2T7 |
|
|
ユーイング肉腫 |
NCC-ES1-C1 |
CVCL_UK81 |
|
NCC-ES2-C1 |
CVCL_B7PH |
|
|
硬化型横紋筋肉腫 |
NCC-ssRMS1-C1 |
CVCL_ZE13 |
|
NCC-ssRMS2-C1 |
CVCL_A0BR |
|
|
多型脂肪肉腫 |
NCC-PLPS1-C1 |
CVCL_A2BR |
|
NCC-PLPS2-C1 |
CVCL_C5Z1 |
|
|
多型肉腫
|
NCC-PS1-C1 |
CVCL_C5Z2 |
|
NCC-PS2-C1 |
Not available |
|
|
悪性ラブドイド腫瘍 |
NCC-MRT1-C1 |
CVCL_C4MW |
|
デスモイド腫瘍 |
NCC-DSM1-C1 |
CVCL_C7G0 |
|
脱分化型軟骨肉腫
|
NCC-dCS1-C1 |
CVCL_UZ21 |
|
NCC-dCS2-C1 |
Not available |
|
| 軟骨肉腫 |
NCC-CS1-C1 |
Not available |
|
明細胞肉腫 |
NCC-CCS1B-C1 |
CVCL_B3TS |
|
NCC-CCS1B-X2-C1 |
CVCL_B3TT |
|
|
NCC-CCS1-X4-C1 |
CVCL_B3TR |
|
|
腱滑膜巨細胞腫 |
NCC-TGCT1-C1 |
CVCL_A9YZ |
|
多型横紋筋肉腫 |
NCC-pRMS1-C1 |
CVCL_B3TM |
|
NCC-pRMS1-X4-C1 |
VCL_B3TN |
|
|
胞巣状横紋筋肉腫
|
NCC-aRMS1-C1 |
CVCL_A1ST |
|
NCC-aRMS2-C1 |
Not available |
|
| 孤立性線維肉腫 |
NCC-SFT1-C1 |
Not available |
Cellosaurus - a knowledge resource on cell lines
Bairoch A. The Cellosaurus, a Cell-Line Knowledge Resource. J Biomol Tech. 2018 Jul;29(2):25-38.
doi: 10.7171/jbt.18-2902-002. Epub 2018 May 10. PMID: 29805321; PMCID: PMC5945021.
樹立した細胞の一覧は、下記のホームページにまとめられています。
NCC Sarcoma Cell Line Panel
https://www.cellosaurus.org/search?query=%22NCC%20sarcoma%20cell%20line%20panel%22